We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
Within the sarcoplasmic reticulum (SR) lumen, CASQ2 polymerizes to form <scene name='56/568018/Dimer/1'>homodimers</scene>, homotetramers and | Within the sarcoplasmic reticulum (SR) lumen, CASQ2 polymerizes to form <scene name='56/568018/Dimer/1'>homodimers</scene>, homotetramers and | ||
<scene name='56/568018/Oligomere_and_ligand/3'>homooligomers</scene>. | <scene name='56/568018/Oligomere_and_ligand/3'>homooligomers</scene>. | ||
| - | There are two | + | There are two forms of dimerization: the |
<scene name='56/568018/Dimer/1'>front-to-front form</scene> and the <scene name='56/568018/Oligomere_and_ligand/5'>back-to-back form</scene>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | <scene name='56/568018/Dimer/1'>front-to-front form</scene> and the <scene name='56/568018/Oligomere_and_ligand/5'>back-to-back form</scene>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | ||
| - | The front-to-front | + | The front-to-front one is stabilized by intermolecular interactions between the |
<scene name='56/568018/Dimer/3'>α2 helix of the domain I</scene> of each CASQ2.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> The intermolecular salt bridges are built between <scene name='56/568018/Dimer/13'>Glu 55 and Lys 49</scene>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> This dimerization induces the formation of an electronegative pocket which involves the following amino acids: Glu 39, Glu 54, Glu 78, Glu 92, Asp 93 and Asp 101 for the first monomere and Glu 199, Asp 245, Asp 278, Glu 348 and Glu 350 for the second one.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | <scene name='56/568018/Dimer/3'>α2 helix of the domain I</scene> of each CASQ2.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> The intermolecular salt bridges are built between <scene name='56/568018/Dimer/13'>Glu 55 and Lys 49</scene>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> This dimerization induces the formation of an electronegative pocket which involves the following amino acids: Glu 39, Glu 54, Glu 78, Glu 92, Asp 93 and Asp 101 for the first monomere and Glu 199, Asp 245, Asp 278, Glu 348 and Glu 350 for the second one.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | ||
| - | The back-to-back form is stabilized by intermolecular interactions between the <scene name='56/568018/Oligomere_and_ligand/7'>α3 helix of the domain I</scene>, <scene name='56/568018/Oligomere_and_ligand/6'>α4 helix of the domain II</scene><ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref>, and it has also been proved that the C-term domain is involved<ref name="c term">NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi</ref> (<scene name='56/568018/Oligomere_and_ligand/9'>together</scene>). The intermolecular salt bridges are built between Glu 215 and Lys 86, Glu 216 and Lys 24, Glu 169 and Lys 85.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> The dimerization is also favored by a hydrogen bond between Ala 82 and Asn 22. This dimerization creates a very electronegative pocket at the C-terminal region which enables the binding of Ca<sup>2+</sup>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> | + | The back-to-back form is stabilized by intermolecular interactions between the <scene name='56/568018/Oligomere_and_ligand/7'>α3 helix of the domain I</scene>, <scene name='56/568018/Oligomere_and_ligand/6'>α4 helix of the domain II</scene><ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref>, and it has also been proved that the <scene name='56/568018/Oligomere_and_ligand/18'>C-term domain</scene> is involved<ref name="c term">NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi</ref> (<scene name='56/568018/Oligomere_and_ligand/9'>together</scene>). The intermolecular salt bridges are built between Glu 215 and Lys 86, Glu 216 and Lys 24, Glu 169 and Lys 85.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> The dimerization is also favored by a hydrogen bond between Ala 82 and Asn 22. This dimerization creates a very electronegative pocket at the C-terminal region which enables the binding of Ca<sup>2+</sup>.<ref name="Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998)">http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html</ref> |
| Line 42: | Line 42: | ||
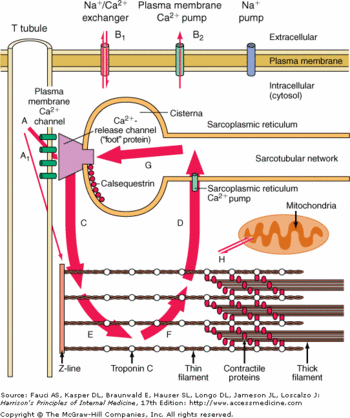
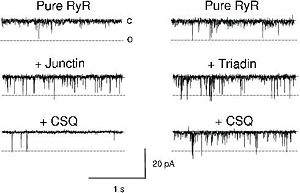
CASQ2 can be anchored into the membrane of SR thanks to two integral proteins: the triadin and the junctin.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">PMID:15731387</ref> Triadin and juctin can bind to CASQ2 on their KEKE motifs (amino acids 210-224 in the triadin chain).<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> Both proteins bind CASQ2 on its Asp-rich region of the C-terminal region.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> | CASQ2 can be anchored into the membrane of SR thanks to two integral proteins: the triadin and the junctin.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">PMID:15731387</ref> Triadin and juctin can bind to CASQ2 on their KEKE motifs (amino acids 210-224 in the triadin chain).<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> Both proteins bind CASQ2 on its Asp-rich region of the C-terminal region.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> | ||
Triadin and Junctin can also interact with Ryanodin Receptor.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> | Triadin and Junctin can also interact with Ryanodin Receptor.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> | ||
| - | The binding site of CASQ2 to Ryanodin Receptor (RyR) is | + | The binding site of CASQ2 to Ryanodin Receptor (RyR) is unknown.<ref name="Regulation of Ryanodine Receptors by Calsequestrin: Effect of High Luminal Ca2+ and Phosphorylation (Beard et Al., 2005)">http://www.ncbi.nlm.nih.gov/pubmed/15731387</ref> |
=== Consequences of CASQ2 binding === | === Consequences of CASQ2 binding === | ||
Revision as of 19:19, 8 January 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ NCBI Gene Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/gene/845
- ↑ Martin JL. Thioredoxin--a fold for all reasons. Structure. 1995 Mar 15;3(3):245-50. PMID:7788290
- ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?ascbin=8&maxaln=10&seltype=2&uid=239372&querygi=429544235&aln=1,227,0,109
- ↑ Polymerization of Calsequestrin: IMPLICATIONS FOR Ca2+ and REGULATION (Park et al., 2003) http://www.jbc.org/content/278/18/16176.full.pdf+html
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi
- ↑ The Asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000) http://www.sciencedirect.com/science/article/pii/S0014579300022468
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004 May;85(1):33-69. PMID:15050380 doi:http://dx.doi.org/10.1016/j.pbiomolbio.2003.07.001
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005 May;88(5):3444-54. Epub 2005 Feb 24. PMID:15731387 doi:http://dx.doi.org/10.1529/biophysj.104.051441
Proteopedia page contributors and editors
Marc-Antoine JACQUES and Thomas VUILLEMIN