We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 814
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Bidding sites == | == Bidding sites == | ||
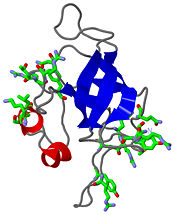
| - | Protein-protein interactions are essential for the stability of ribosomes. The L14 subunits presents a perfect hydrophobic area on its structure too allow such an interaction with other ribosome’s subunits. This area is on the beta-barrel and is composed of <scene name='56/568012/Hydrophobarea/3'>four residues</scene> : Leu25, Val40, Val57 and Ile2. This area is very exposed and separated from the RNA binding site. | + | Protein-protein interactions are essential for the stability of ribosomes. The L14 subunits presents a perfect hydrophobic area on its structure too allow such an interaction with other ribosome’s subunits. This area is on the beta-barrel and is composed of <scene name='56/568012/Hydrophobarea/3'>four residues</scene> : Leu25, Val40, Val57 and Ile2. This area is very exposed and separated from the RNA binding site. Links to L4, L7/L12, L10, L11, L17 and L19 has been showed and are allowed by this hydrophobic structure. |
== Role of the L14 subunit == | == Role of the L14 subunit == | ||
Revision as of 21:41, 8 January 2014
RIBOSOMAL PROTEIN L14
| |||||||||||
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |