Journal:BAMBEd:Acetylcholinesterase: Substrate Traffic and Inhibition
From Proteopedia
(Difference between revisions)

| Line 14: | Line 14: | ||
==='''Background Information'''=== | ==='''Background Information'''=== | ||
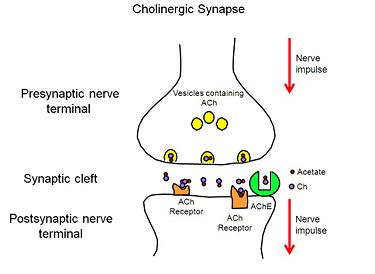
| - | [[Image:AChE-Page-Cholinergic-Synapse.jpg|thumb|alt= Alt text| Figure 2. Cholinergic Synapse |375px]] | + | [[Image:AChE-Page-Cholinergic-Synapse.jpg|left|thumb|alt= Alt text| Figure 2. Cholinergic Synapse |375px]] |
| - | + | {{clear}} | |
When a nerve impulse reaches the presynaptic nerve terminal of a cholinergic synpase, it stimulates the release of the neurotransmitter, ACh (Figure 1), into the synaptic cleft. ACh diffuses across the cleft to the postsynaptic nerve terminal, where it binds reversibly to acetylcholine receptors embedded in the membrane of the postsynaptic nerve terminal. The binding of ACh to the receptors triggers a nerve impulse in the postsynaptic neuron. Finally AChE, anchored to the membrane of the postsynaptic nerve terminal (Figure 2), hydrolyzes ACh to acetate and choline, resulting in the termination of neurotransmission. | When a nerve impulse reaches the presynaptic nerve terminal of a cholinergic synpase, it stimulates the release of the neurotransmitter, ACh (Figure 1), into the synaptic cleft. ACh diffuses across the cleft to the postsynaptic nerve terminal, where it binds reversibly to acetylcholine receptors embedded in the membrane of the postsynaptic nerve terminal. The binding of ACh to the receptors triggers a nerve impulse in the postsynaptic neuron. Finally AChE, anchored to the membrane of the postsynaptic nerve terminal (Figure 2), hydrolyzes ACh to acetate and choline, resulting in the termination of neurotransmission. | ||
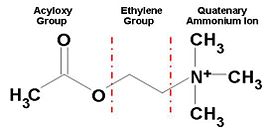
[[Image:AChE-Page-ACh_shematic.JPG|left|thumb|alt= Alt text| Figure 1. Chemical Structure of Acetylcholine |275px]] | [[Image:AChE-Page-ACh_shematic.JPG|left|thumb|alt= Alt text| Figure 1. Chemical Structure of Acetylcholine |275px]] | ||
| - | + | {{clear}} | |
Inhibition of AChE may result in various outcomes, depending on the physiological context. Toxins such as FAS-II, from the green mamba, a poisonous snake found in East Africa, inhibit AChE and ultimately lead to death. However, controlled inhibition of AChE, in patients with Alzheimer’s disease, by drugs designed for this purpose, alleviates their symptoms, including memory loss and disorientation. | Inhibition of AChE may result in various outcomes, depending on the physiological context. Toxins such as FAS-II, from the green mamba, a poisonous snake found in East Africa, inhibit AChE and ultimately lead to death. However, controlled inhibition of AChE, in patients with Alzheimer’s disease, by drugs designed for this purpose, alleviates their symptoms, including memory loss and disorientation. | ||
| Line 44: | Line 44: | ||
---- | ---- | ||
<scene name='Sandbox_250/Ache_ach/1'>AChE/ACh</scene> | <scene name='Sandbox_250/Ache_ach/1'>AChE/ACh</scene> | ||
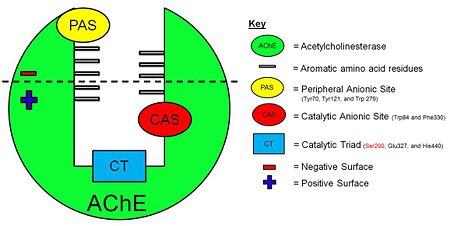
| - | [[Image:AChE-Page-schematic-gorge.jpg|thumb|alt= Alt text| Figure 3. Schematic illustration of AChE. | | + | [[Image:AChE-Page-schematic-gorge.jpg|left|thumb|alt= Alt text| Figure 3. Schematic illustration of AChE. |450px]] |
| - | + | {{clear}} | |
The ''Tc''<scene name='Sandbox_250/Ache_ach/5'>AChE</scene> protein contains 537 amino acids and forms an α/β hydrolase fold. The neurotransmitter <scene name='Sandbox_250/Ache_ach/36'>ACh</scene> consists of an acytoxy group, an ethylene group and a positively charged quaternary ammonium ion. | The ''Tc''<scene name='Sandbox_250/Ache_ach/5'>AChE</scene> protein contains 537 amino acids and forms an α/β hydrolase fold. The neurotransmitter <scene name='Sandbox_250/Ache_ach/36'>ACh</scene> consists of an acytoxy group, an ethylene group and a positively charged quaternary ammonium ion. | ||
Revision as of 08:44, 26 February 2014
| |||||||||||
References
- ↑ Greenblatt HM, Dvir H, Silman I, Sussman JL. Acetylcholinesterase: a multifaceted target for structure-based drug design of anticholinesterase agents for the treatment of Alzheimer's disease. J Mol Neurosci. 2003;20(3):369-83. PMID:14501022 doi:10.1385/JMN:20:3:369
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
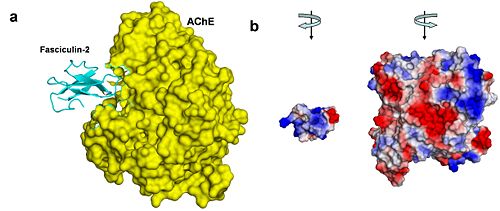
- ↑ Harel M, Kleywegt GJ, Ravelli RB, Silman I, Sussman JL. Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure. 1995 Dec 15;3(12):1355-66. PMID:8747462
- ↑ Silman I, Sussman JL. Acetylcholinesterase: how is structure related to function? Chem Biol Interact. 2008 Sep 25;175(1-3):3-10. Epub 2008 Jun 6. PMID:18586019 doi:10.1016/j.cbi.2008.05.035
- ↑ Kessel A and Ben-Tal N (Dec. 2010) Introduction to Proteins: Structure, Function, and Motion. Chapman & Hall/CRC Mathematical & Computational Biology. ISBN: 9781439810712
Acknowledgements
1. Howard Hughes Medical Institue Pre-College Program
2. Center for BioMolecular Modeling, Milwaukee School of Engineering
3. The Rockefeller University Center for Clinical and Translational Science
4. The Rockefeller University S.M.A.R.T Team Program
5. The Rockefeller University Science Outreach Program
6. Touro College of Pharmacy
7. Michal Harel, Weizmann Institute of Science
8. Natural Sciences Department,Hostos Community College, Bronx, NY
9. Malcolm Twist
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Michal Harel, Daviana Dueno, Randol Mata, Mary Acheampong, Allison Granberry, Alafia Henry, Marisa L. VanBrakle
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.