We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 914
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
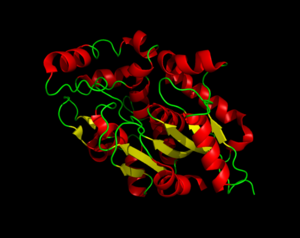
== Structure == | == Structure == | ||
| - | The secondary structure of PPT1 contains several α-helices and few β-sheets. | + | The secondary structure of PPT1 contains several α-helices and few β-sheets. |
| + | |||
=== α/β Hydrolase Fold === | === α/β Hydrolase Fold === | ||
| - | The α/β Hydrolase Fold is common to many other hydrolases. This fold consists of β3-β8, αA, αB, αC, and αF. | + | The α/β Hydrolase Fold is common to many other hydrolases. This fold consists of β3-β8, αA, αB, αC, and αF. The α/β hydrolase fold has a central 6 stranded parallel Beta sheet. There is also a helix that is roughly perpendicular to the direction of the beta sheet. There is also a large insertion, containing 6 helices, that forms a second domain that encompasses most of the fatty acid binding site. |
=== Catalytic Triad === | === Catalytic Triad === | ||
| - | + | The catalytic triad is composed of Ser115, His289, and Asp233, which is the same as the catalytic triad in chymotrypsin. | |
| + | A water molecule is occupying the oxyanion hole and it is hydrogen bonded to ser115. | ||
| + | |||
===Hydrophobic Groove === | ===Hydrophobic Groove === | ||
| + | The hydrophobic binding groove is located in the second domain of PPT1, where palmitate mainly binds. The fact that palmitate has to bend to fit into the binding pocket suggests that this pocket is designed to bind an unsaturated fatty acid. | ||
== Function == | == Function == | ||
===Biological === | ===Biological === | ||
Revision as of 13:35, 18 March 2014
Palmitoyl-Protein Thioesterase 1
| |||||||||||