We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 914
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
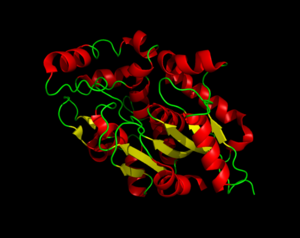
[[Image:sandbox1.png|300px|left|thumb|Three dimensional structure of Palmitoyl-Protein Thioesterase 1.]] | [[Image:sandbox1.png|300px|left|thumb|Three dimensional structure of Palmitoyl-Protein Thioesterase 1.]] | ||
| - | Palmitoyl-Protein Thioesterase 1 (PPT1) is a lysosomal enzyme that plays a role in the degradation of lipid-modified proteins<ref name="human">Palmitoyl-Protein Thioesterase 1 Precursor - Homo Sapiens. N.p., 1 Oct. 1996.</ref>. PPT1 receives its catalytic power from its catalytic triad, the α/β hydrolase fold, and its hydrophobic groove in order to remove fatty acid acyl groups, typically palmitate from cysteine residues in proteins. PPT1 demonstrates how proteins can be modified by different enzymes and induce biological changes. Misregulation of PPT1 modifications can cause various diseases, including infantile neuronal ceroid lipofuscinosis, kufs disease, and late-infantile neuronal ceroid lipofuscinosis. Within these diseases, the production of PPT1 is decreased or eliminated completely, which leads to fatty acid buildup. | + | Palmitoyl-Protein Thioesterase 1 (PPT1) is a lysosomal enzyme that plays a role in the degradation of lipid-modified proteins<ref name="human">Palmitoyl-Protein Thioesterase 1 Precursor - Homo Sapiens. N.p., 1 Oct. 1996.</ref>. PPT1 receives its catalytic power from its catalytic triad, the α/β hydrolase fold, and its hydrophobic groove in order to remove fatty acid acyl groups, typically palmitate from cysteine residues in proteins. PPT1 demonstrates how proteins can be modified by different enzymes and induce biological changes <ref name="human"/>. Misregulation of PPT1 modifications can cause various diseases, including infantile neuronal ceroid lipofuscinosis, kufs disease, and late-infantile neuronal ceroid lipofuscinosis. Within these diseases, the production of PPT1 is decreased or eliminated completely, which leads to fatty acid buildup <ref name="PPT"/>. |
== Structure == | == Structure == | ||
| - | The secondary structure of PPT1 contains several α-helices and few β-sheets. PPT1 includes residues 28-306, after the 27-residue signal peptide has been removed. There is a large insertion between β6 and β7, residues 140-223, and that forms a second domain that is compromised almost entirely of the fatty acid binding site. This second domain region contains six helices, α2-α7. | + | The secondary structure of PPT1 contains several α-helices and few β-sheets. PPT1 includes residues 28-306, after the 27-residue signal peptide has been removed <ref name="RSCB">Bellizzi III, John. RCSB Protein Data Bank. PNAS, 18 Apr. 2000.</ref>. There is a large insertion between β6 and β7, residues 140-223, and that forms a second domain that is compromised almost entirely of the fatty acid binding site. This second domain region contains six helices, α2-α7<ref name="RSCB"/>. |
=== α/β Hydrolase Fold === | === α/β Hydrolase Fold === | ||
| Line 34: | Line 34: | ||
PPT1 mutations are the root cause of several diseases, specifically those where mutations cause a decrease or depletion of PPT1. Infantile neuronal ceroid lipofuscinosis (INCF) is characterized by impaired mental and motor development, including difficulty with walking, speaking, and intellectual function, beginning around the first or second year of life<ref name="PPT">PPT1. Genetics Home Reference. U.S. National Library of Medicine, Aug. 2015.</ref>. The PPT1 mutation involved in INCF replaces an arginine with a stop signal in the instructions to make the enzyme. This mutation leads to a vast reduction in the production of PPT1, which impairs the removal of fatty acids from proteins. This impaired removal leads to fatty acid accumulations throughout the body, particularly in neuronal cells in the brain<ref name="PPT"/>. Late-infantile neuronal ceroid lipofuscinosis has the same characteristics as INCF, but the mutation varies slightly. This leads to a slight reduction in the activity of PPT1 instead of completely wiping it out. | PPT1 mutations are the root cause of several diseases, specifically those where mutations cause a decrease or depletion of PPT1. Infantile neuronal ceroid lipofuscinosis (INCF) is characterized by impaired mental and motor development, including difficulty with walking, speaking, and intellectual function, beginning around the first or second year of life<ref name="PPT">PPT1. Genetics Home Reference. U.S. National Library of Medicine, Aug. 2015.</ref>. The PPT1 mutation involved in INCF replaces an arginine with a stop signal in the instructions to make the enzyme. This mutation leads to a vast reduction in the production of PPT1, which impairs the removal of fatty acids from proteins. This impaired removal leads to fatty acid accumulations throughout the body, particularly in neuronal cells in the brain<ref name="PPT"/>. Late-infantile neuronal ceroid lipofuscinosis has the same characteristics as INCF, but the mutation varies slightly. This leads to a slight reduction in the activity of PPT1 instead of completely wiping it out. | ||
Mutations can also cause premature stop signals to be added to the instructions to create PPT1, resulting in Kufs disease. This is characterized by seizures, problems with movement, and a decline of intellectual function, usually beginning in early adulthood<ref name="PPT"/>. Although premature stop signals are added, these mutations allow enough PPT1 to be produced so that the onset is later on in life and the life expectancy is higher. | Mutations can also cause premature stop signals to be added to the instructions to create PPT1, resulting in Kufs disease. This is characterized by seizures, problems with movement, and a decline of intellectual function, usually beginning in early adulthood<ref name="PPT"/>. Although premature stop signals are added, these mutations allow enough PPT1 to be produced so that the onset is later on in life and the life expectancy is higher. | ||
| - | |||
</StructureSection> | </StructureSection> | ||
Revision as of 03:23, 1 April 2014
ββ
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
Palmitoyl-Protein Thioesterase 1
| |||||||||||
References
- ↑ 1.0 1.1 Palmitoyl-Protein Thioesterase 1 Precursor - Homo Sapiens. N.p., 1 Oct. 1996.
- ↑ 2.0 2.1 2.2 2.3 PPT1. Genetics Home Reference. U.S. National Library of Medicine, Aug. 2015.
- ↑ 3.0 3.1 Bellizzi III, John. RCSB Protein Data Bank. PNAS, 18 Apr. 2000.
External Resources
http://en.wikipedia.org/wiki/Palmitoyl_protein_thioesterase
https://www.counsyl.com/diseases/ppt1-related-neuronal-ceroid-lipofuscinosis/
http://en.wikipedia.org/wiki/Alpha/beta_hydrolase_fold
http://en.wikipedia.org/wiki/Catalytic_triad
http://www.biomedcentral.com/1471-2121/8/22
http://www.genecards.org/cgi-bin/carddisp.pl?gene=PPT1