Phosphofructokinase (PFK)
From Proteopedia
| Line 1: | Line 1: | ||
| - | <StructureSection load='4pfk' scene='Phosphofructokinase_(PFK)/4pfk_biol/3' size='400 | + | <StructureSection load='4pfk' scene='Phosphofructokinase_(PFK)/4pfk_biol/3' size='400' |
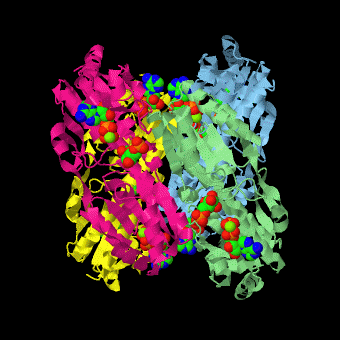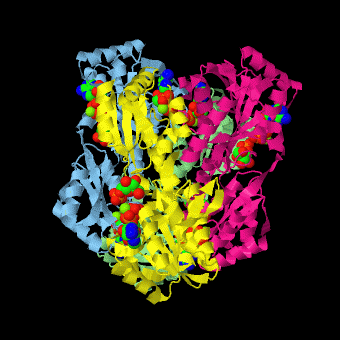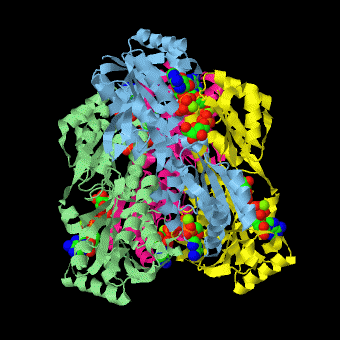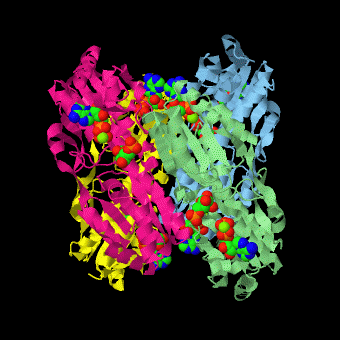
caption='PFK: R-state Biological tetramer complex with fructose-6-phosphate, ADP and Mg+2 ion; generated from [[4pfk]] by QPS' > | caption='PFK: R-state Biological tetramer complex with fructose-6-phosphate, ADP and Mg+2 ion; generated from [[4pfk]] by QPS' > | ||
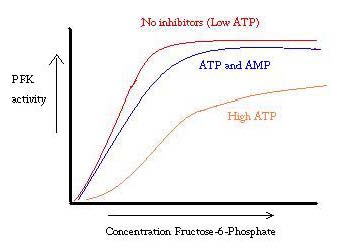
'''Phosphofructokinase-1''' (PFK-1) is a glycolytic enzyme that catalyzes the transfer of a phosphoryl group from <scene name='Phosphofructokinase_(PFK)/Cv/21'>ATP</scene> to <scene name='Phosphofructokinase_(PFK)/Cv/22'>fructose-6-phosphate (F6P)</scene> to yield <scene name='Phosphofructokinase_(PFK)/Cv/16'>ADP</scene> and <scene name='Phosphofructokinase_(PFK)/Cv/23'>fructose-1,6-bisphosphate (FBP)</scene>. Mg2+ is also important in this reaction (<scene name='Phosphofructokinase_(PFK)/Cv/24'>click here to see animation of reaction</scene>). '''Phosphofructokinase-2''' (PFK-2) acts on the same substrates to yield ADP and <scene name='Phosphofructokinase_(PFK)/Cv1/3'>fructose-2,6-bisphosphate (F2,6P)</scene>. <scene name='Phosphofructokinase_(PFK)/Cv1/4'>Click here to see the difference between FBP and F2,6P</scene>. PFK reaction is strongly exergonic (irreversible) under physiological conditions and hence is one of the glycolytic pathway's rate-determining steps. In most organisms/tissues, PFK is the glycolytic pathway's major flux-regulating enzyme; its activity is controlled by the concentrations of an unusually large number of metabolites including ATP, ADP, AMP, PEP and fructose-2,6-bisphosphate. | '''Phosphofructokinase-1''' (PFK-1) is a glycolytic enzyme that catalyzes the transfer of a phosphoryl group from <scene name='Phosphofructokinase_(PFK)/Cv/21'>ATP</scene> to <scene name='Phosphofructokinase_(PFK)/Cv/22'>fructose-6-phosphate (F6P)</scene> to yield <scene name='Phosphofructokinase_(PFK)/Cv/16'>ADP</scene> and <scene name='Phosphofructokinase_(PFK)/Cv/23'>fructose-1,6-bisphosphate (FBP)</scene>. Mg2+ is also important in this reaction (<scene name='Phosphofructokinase_(PFK)/Cv/24'>click here to see animation of reaction</scene>). '''Phosphofructokinase-2''' (PFK-2) acts on the same substrates to yield ADP and <scene name='Phosphofructokinase_(PFK)/Cv1/3'>fructose-2,6-bisphosphate (F2,6P)</scene>. <scene name='Phosphofructokinase_(PFK)/Cv1/4'>Click here to see the difference between FBP and F2,6P</scene>. PFK reaction is strongly exergonic (irreversible) under physiological conditions and hence is one of the glycolytic pathway's rate-determining steps. In most organisms/tissues, PFK is the glycolytic pathway's major flux-regulating enzyme; its activity is controlled by the concentrations of an unusually large number of metabolites including ATP, ADP, AMP, PEP and fructose-2,6-bisphosphate. | ||
Revision as of 06:20, 20 August 2014
| |||||||||||
Contents |
3D structures of PFK
Updated on 20-August-2014
Phosphofructokinase
3o8l, 3o8n – PFK – rabbit
3o8o – PFK – yeast
2pfk – EcPFK-1 – Escherichia coli
3umo – EcPFK-2
2hig – TbPFK – Trypanosoma brucei
1zxx – PFK – Lactobacillus delbrueckii
6pfk, 3pfk, 3u39 – GsPFK – Geobacillus stearothermophilus
4i36 – GsPFK (mutant)
1u2x – PhPFK – Pyrococcus horikoshii
3hic – LiPFK – Listeria innocua
3opy – PFK – Pichia pastoris
4du5 – PFKB - Polaromonas
4a3s – PFK – Bacillus subtilis
PFK complexes
3n1c – EcPFK-2 + fructose-6-phosphate
3cqd, 3ump – EcPFK-2 + ATP
3uqe - EcPFK2 (mutant) + ATP
3uqd – EcPFK2 + fructose-1,6-bisphosphate + ATP
1pfk – EcPFK + fructose-1,6-bisphosphate + ADP
3drw – PhPFK (mutant) + ADP
3f5m – TbPFK + ATP
3ie7 – LiPFK + ATP
1mto - GsPFK (mutant) + fructose-6-phosphate
4i4i, 4i7e - GsPFK (mutant) + phosphoenolpyruvate
6pfk – GsPFK + phosphoglycolic acid
4pfk - GsPFK + fructose-6-phosphate + ADP
Pyrophosphate-dependent PFK
1kzh – PFK – Borrelia burgdorferi
3k2q – PFK – Marinobacter aquaeolei
3hno – PFK – Nitrosospira multiformis
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Schirmer T, Evans PR. Structural basis of the allosteric behaviour of phosphofructokinase. Nature. 1990 Jan 11;343(6254):140-5. PMID:2136935 doi:http://dx.doi.org/10.1038/343140a0
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Evans PR, Farrants GW, Hudson PJ. Phosphofructokinase: structure and control. Philos Trans R Soc Lond B Biol Sci. 1981 Jun 26;293(1063):53-62. PMID:6115424
- ↑ http://www.nature.com/nature/journal/v327/n6121/abs/327437a0.html
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ PubMed:2136935
- ↑ Campos G, Guixe V, Babul J. Kinetic mechanism of phosphofructokinase-2 from Escherichia coli. A mutant enzyme with a different mechanism. J Biol Chem. 1984 May 25;259(10):6147-52. PMID:6233271
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ PubMed:2136935
- ↑ Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008. Print.
- ↑ Campos G, Guixe V, Babul J. Kinetic mechanism of phosphofructokinase-2 from Escherichia coli. A mutant enzyme with a different mechanism. J Biol Chem. 1984 May 25;259(10):6147-52. PMID:6233271
- ↑ Kimmel JL, Reinhart GD. Reevaluation of the accepted allosteric mechanism of phosphofructokinase from Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):3844-9. PMID:10759544 doi:10.1073/pnas.050588097
External Links
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Judy Voet, Ann Taylor, Jaime Prilusky, David Canner, Eran Hodis, Joel L. Sussman, Zach Westrick