We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox reserved 919
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
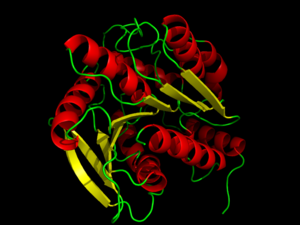
[[Image:Proteopediapic.png|300 px|left|thumb|Hormone-Sensitive Lipase Complex with PMSF from [http://proteopedia.org/wiki/index.php/3h17 3h17]]] | [[Image:Proteopediapic.png|300 px|left|thumb|Hormone-Sensitive Lipase Complex with PMSF from [http://proteopedia.org/wiki/index.php/3h17 3h17]]] | ||
| - | Hormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride ([http://en.wikipedia.org/wiki/PMSF PMSF]) | + | Hormone-sensitive lipase can be inhibited by phenylmethylsufonyl flouride ([http://en.wikipedia.org/wiki/PMSF PMSF]) binding to the <scene name='58/580296/Meshligand/2'>active site</scene>. The experiments performed to test this inhibition used different strains of bacteria, which had a different order of residues but the same catalytic effect. This experiment tested the Ser144 residue rather than the Ser157 residue seen in other HSL proteins. PMSF inhibits enzymes by binding to the Ser144 residue of the [http://proteopedia.org/wiki/index.php/Serine_Proteases serine protease] active sites so that the normal catalytic activity cannot be carried out. This inhibitor will only bind to the active site Ser144 because of its participation in the charge relay of the <scene name='58/580296/Meshligandinteraction/1'>catalytic triad</scene>. This hyper activity allows the sulfonyl group of PMSF to <scene name='58/580296/Inhibitorinteraction/4'>covalently bond</scene> to the Ser144 residue to disrupt its activity. Because of this Ser144 residue specificity, PMSF does not inhibit all kinds of lipases, just those dependent on Ser144 residues in the active site. PMSF is highly degradable in aqueous solutions so it does not inhibit for very long periods of time in its natural environment. PMSF binding induces only a minor conformational change from the native protein.<ref name="Kanwar">PMID:23923547</ref> |
Inhibition or decreased activity of hormone-sensitive lipases can possibly lead to disorders such as atherosclerosis, obesity, and type 2 diabetes. Inability to break down fatty acid molecules due to inactive hormone-sensitive lipases has been reported in many cases of obesity and type 2 diabetes. Increased inhibition of HSL by PMSF could, in theory, also be a possible cause of these diseases.<ref name="Kraemer">PMID: 12364542 </ref> | Inhibition or decreased activity of hormone-sensitive lipases can possibly lead to disorders such as atherosclerosis, obesity, and type 2 diabetes. Inability to break down fatty acid molecules due to inactive hormone-sensitive lipases has been reported in many cases of obesity and type 2 diabetes. Increased inhibition of HSL by PMSF could, in theory, also be a possible cause of these diseases.<ref name="Kraemer">PMID: 12364542 </ref> | ||
Revision as of 15:36, 21 April 2014
| |||||||||||
Additional pages about hormone-sensitive lipase
References
- ↑ 1.0 1.1 Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. PMID:14641008 doi:http://dx.doi.org/10.1042/
- ↑ 2.0 2.1 Ray H, Beylot M, Arner P, Larrouy D, Langin D, Holm C, Large V. The presence of a catalytically inactive form of hormone-sensitive lipase is associated with decreased lipolysis in abdominal subcutaneous adipose tissue of obese subjects. Diabetes. 2003 Jun;52(6):1417-22. PMID:12765952
- ↑ 3.0 3.1 3.2 3.3 Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004 Apr 1;379(Pt 1):11-22. PMID:14725507 doi:http://dx.doi.org/10.1042/BJ20031811
- ↑ 4.0 4.1 4.2 4.3 Nam KH, Kim MY, Kim SJ, Priyadarshi A, Kwon ST, Koo BS, Yoon SH, Hwang KY. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins. 2009 Mar;74(4):1036-40. PMID:19089974 doi:http://dx.doi.org/10.1002/prot.22313
- ↑ Kanwar SS, Kaushal RK, Jawed A, Gupta R, Chimni SS. Methods for inhibition of residual lipase activity in colorimetric assay: a comparative study. Indian J Biochem Biophys. 2005 Aug;42(4):233-7. PMID:23923547
- ↑ Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002 Oct;43(10):1585-94. PMID:12364542