We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Brittany Carroll/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
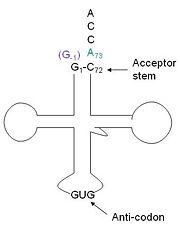
[[Image:Trnahis.jpg|left|thumb|tRNA His, highlighting the incoming G-1 (purple) opposite A73 (green)]] | [[Image:Trnahis.jpg|left|thumb|tRNA His, highlighting the incoming G-1 (purple) opposite A73 (green)]] | ||
| - | [http://www. | + | [http://www.en.wikipedia.org/wiki/Transfer_RNA tRNA]His has a [http://www.en.wikipedia.org/wiki/Guanosine_monophosphate guanine monophosphate] (GMP) residue at the 5’ end in all domains of life, besides α-proteopbacteria. This GMP is referred to as G-1. In prokaryotes G-1 is encoded in the genome. [http://www.en.wikipedia.org/wiki/RNase_P RNase P] cleaves pre-tRNAHis to generate the mature tRNA, leaving an extra basepair on the acceptor stem, G-1:C73. In eukaryotes the G-1 residue is not encoded and needs to be added post-transcription. The enzyme that catalyzes this reaction is the polymerase,[http://www.en.wikipedia.org/wiki/Guanylyl_transferase tRNAHis guanylyltransferase] (Thg1). Howerver, the addition of the GMP residue is nontemplated, inserting GMP across from A73 in the acceptor stem creating a mismatch. Unlike most polymerases, Thg1 adds nucleotides in the 3’ –to- 5’ direction, while forming a normal 3’ –to- 5’ phosphodiester bond. Therefore, the 3’-OH of the incoming nucleotide attacks the 5’ end of the polynucleotide chain. This is a two step mechanism where the polynucleotide chain is first adenylated and then guanylated. |
This addition is interesting for multiple reasons. It is one of only a few known reactions where a normal 3’-to-5’ phosphodiester bond is formed in a 3’ –to- 5’ direction. Also, the additional 5’ nucleotide is unique to tRNAHis, with the exception of a tRNAPhe species. Lastly, this modification is essential, at least in yeast. | This addition is interesting for multiple reasons. It is one of only a few known reactions where a normal 3’-to-5’ phosphodiester bond is formed in a 3’ –to- 5’ direction. Also, the additional 5’ nucleotide is unique to tRNAHis, with the exception of a tRNAPhe species. Lastly, this modification is essential, at least in yeast. | ||
| Line 16: | Line 16: | ||
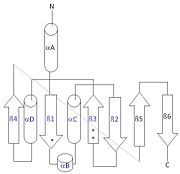
[[Image:thg1topology.jpg|right|thumb| This 2D topology diagram shows the βαββαβ fold of Thg1. The helices and strands involved in the fold are in blue font. The fold is most similar to that of cylcases. The mechanism is more likely the same as family A polymerases, with the conserved carboxylates shown as asterisks(*).]] | [[Image:thg1topology.jpg|right|thumb| This 2D topology diagram shows the βαββαβ fold of Thg1. The helices and strands involved in the fold are in blue font. The fold is most similar to that of cylcases. The mechanism is more likely the same as family A polymerases, with the conserved carboxylates shown as asterisks(*).]] | ||
| - | Interestingly, Thg1 shares structural similarities to both cyclases and the palm domain of canonical polymerases, without sequence similarities. The βαββαβ motif is most homologous with adenylyl and guanylyl cyclases. However, based upon the model the mechanism seems to be more like that of a family A polymerase. The model suggests Thg1 has three catalytic carboxylates: aspartate 29, aspartate 76, and glutamate 77. Cyclases only have two catalytic carboxylates, two aspartates and either cysteine, alanine, or glycine. The position of the carboxylates in Thg1 is homologous to those of T7 DNA Polymerase. An overlay of the palm domain of T7 and Thg1 shows that the three carboxylates, two metal ions, and the incoming nucleotide are conserved and in similar postions. This indicates that Thg1 most likely uses the two-metal-ion mechaism of canonical 5' to 3' polymerases. | + | Interestingly, Thg1 shares structural similarities to both [http://www.en.wikipedia.org/wiki/Cyclase cyclases] and the palm domain of canonical [http://www.en.wikipedia.org/wiki/DNA_polymerase polymerases], without sequence similarities. The βαββαβ motif is most homologous with adenylyl and guanylyl cyclases. However, based upon the model the mechanism seems to be more like that of a family A polymerase. The model suggests Thg1 has three catalytic carboxylates: aspartate 29, aspartate 76, and glutamate 77. Cyclases only have two catalytic carboxylates, two aspartates and either cysteine, alanine, or glycine. The position of the carboxylates in Thg1 is homologous to those of [http://www.en.wikipedia.org/wiki/T7_DNA_polymerase T7 DNA Polymerase]. An overlay of the palm domain of T7 and Thg1 shows that the three carboxylates, two metal ions, and the incoming nucleotide are conserved and in similar postions. This indicates that Thg1 most likely uses the two-metal-ion mechaism of canonical 5' to 3' polymerases. |
== Structure == | == Structure == | ||
Revision as of 02:12, 27 April 2014
tRNA(His) guanylyltransferase
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644