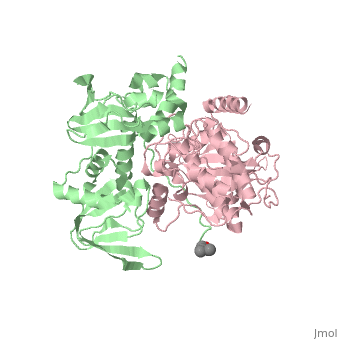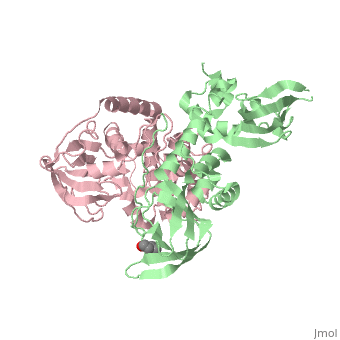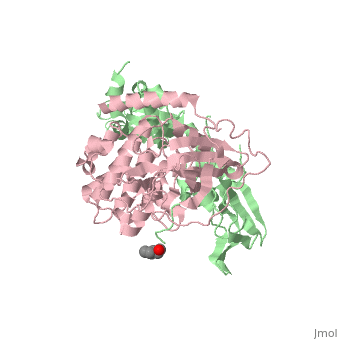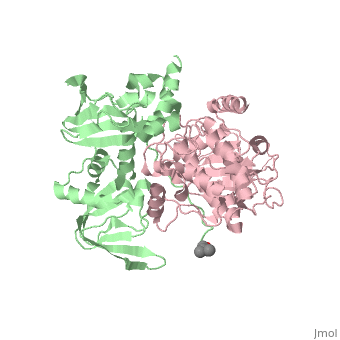We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Protein Kinase A
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
| - | PKA consists of two types of subunits. It has a 49-kd | + | PKA consists of two types of subunits. It has a 49-kd <scene name='58/582909/Be-rs/2'>regulatory subunit</scene> (R) and a 38-kd <scene name='58/582909/Real_cs/1'>catalytic subunit</scene> (C). The R dimer contains two cAMP bindng domains and the pseudosubstrate sequence, Arg-Arg-Gly-Ala-Ile. The C subunit has two lobes. The larger lobe binds the peptide and contributes the key catalytic residues and the smaller lobe binds ATP-Mg2+. This <scene name='58/582909/Catalytic_subunit_1/1'>scene</scene> shows a catalytic subunit with an inhibitor with a <scene name='58/582909/Psuedosubstrate/1'>pseudosubstrate</scene> bound to the active site and an ATP bound to the adjacent active site. When PKA is enzymatically inactive (absence of cAMP), it is a heterotetrameric complex of a regulatory dimer bound to two catalytic subunits, forming the R2C2 complex. In its active state, the complex dissociates to form an R2 subunit and two C subunits. The C subunits are enzymatically active in itself and once released, are free to bind and phosphorylate available substrate proteins. |
== Mechanism == | == Mechanism == | ||
Revision as of 20:36, 2 May 2014
Protein kinase A (PKA)
| |||||||||||
References
[1]Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644 [2]C. Kim, N.-H. Xuong, and S.S. Taylor, "Crystal structure of a complex between catalytic and regulatory (Rlα) subunits of PKA,"Science 307, 690 (2005) doi:10.2210/rcsb_pdb/mom_2012_8