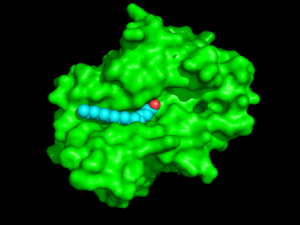
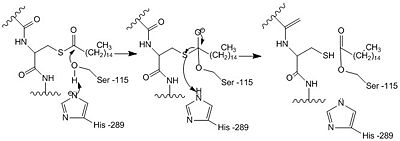
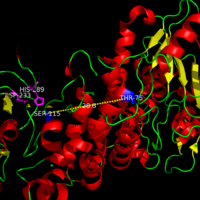
Palmitoyl protein thioesterase
From Proteopedia
(Difference between revisions)
| Line 58: | Line 58: | ||
===Mutations leading to Juvenile Neuronal Ceroid Lipofuscinosis=== | ===Mutations leading to Juvenile Neuronal Ceroid Lipofuscinosis=== | ||
JNCL is caused by a mutation in the CLN3 gene which codes for a lysosomal membrane protein of unknown function <ref name="Ryan-3">PMID:9151311</ref>. Unlike the mutations that cause INCL and LINCL, mutations that lead to JNCL are located away from the active site and are seen to cause less damage to the overall structure of PPT-1. Some of the mutations in JNCL have been noted as retaining a low level of PPT-1 activity as the catalytic site is left fairly unperturbed. Mutations associated with JNCl are found in two locations, Thr75Pro with Asp79Gly and <scene name='58/580837/Juvenile_mutation/4'>Tyr247His with Gly250Val</scene>. These mutations occur in conjugate with its other pair and are predicted to disturb the geometry of helix α1, increasing the flexibility of the region, and alter the antiparallel βsheet motif in sheets βa and βb compared to the <scene name='58/580837/Tyrosine_normal/2'>normal Tyr-247 and Gly-250</scene>. | JNCL is caused by a mutation in the CLN3 gene which codes for a lysosomal membrane protein of unknown function <ref name="Ryan-3">PMID:9151311</ref>. Unlike the mutations that cause INCL and LINCL, mutations that lead to JNCL are located away from the active site and are seen to cause less damage to the overall structure of PPT-1. Some of the mutations in JNCL have been noted as retaining a low level of PPT-1 activity as the catalytic site is left fairly unperturbed. Mutations associated with JNCl are found in two locations, Thr75Pro with Asp79Gly and <scene name='58/580837/Juvenile_mutation/4'>Tyr247His with Gly250Val</scene>. These mutations occur in conjugate with its other pair and are predicted to disturb the geometry of helix α1, increasing the flexibility of the region, and alter the antiparallel βsheet motif in sheets βa and βb compared to the <scene name='58/580837/Tyrosine_normal/2'>normal Tyr-247 and Gly-250</scene>. | ||
| + | =3D structures of palmitoyl protein thioesterase= | ||
| + | [[Thioesterase]] | ||
</StructureSection> | </StructureSection> | ||
Revision as of 09:11, 31 December 2014
| |||||||||||
References
- ↑ Wang R, Borazjani A, Matthews AT, Mangum LC, Edelmann MJ, Ross MK. Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme. Biochemistry. 2013 Oct 29;52(43):7559-74. doi: 10.1021/bi401138s. Epub 2013 Oct, 18. PMID:24083319 doi:http://dx.doi.org/10.1021/bi401138s
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Bellizzi JJ 3rd, Widom J, Kemp C, Lu JY, Das AK, Hofmann SL, Clardy J. The crystal structure of palmitoyl protein thioesterase 1 and the molecular basis of infantile neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4573-8. PMID:10781062 doi:10.1073/pnas.080508097
- ↑ 3.0 3.1 Simonati A, Tessa A, Bernardina BD, Biancheri R, Veneselli E, Tozzi G, Bonsignore M, Grosso S, Piemonte F, Santorelli FM. Variant late infantile neuronal ceroid lipofuscinosis because of CLN1 mutations. Pediatr Neurol. 2009 Apr;40(4):271-6. doi: 10.1016/j.pediatrneurol.2008.10.018. PMID:19302939 doi:http://dx.doi.org/10.1016/j.pediatrneurol.2008.10.018
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Bellizzi JJ 3rd, Widom J, Kemp C, Lu JY, Das AK, Hofmann SL, Clardy J. The crystal structure of palmitoyl protein thioesterase 1 and the molecular basis of infantile neuronal ceroid lipofuscinosis. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4573-8. PMID:10781062 doi:10.1073/pnas.080508097
- ↑ 5.0 5.1 5.2 Branneby, Cecilia. "Exploiting Enzyme Promiscuity for Rational Design." KTH Biotechnology (2005): Web. 10 Apr. 2013.
- ↑ 6.0 6.1 Hofmann SL, Das AK, Yi W, Lu JY, Wisniewski KE. Genotype-phenotype correlations in neuronal ceroid lipofuscinosis due to palmitoyl-protein thioesterase deficiency. Mol Genet Metab. 1999 Apr;66(4):234-9. PMID:10191107 doi:http://dx.doi.org/10.1006/mgme.1999.2803
- ↑ 7.0 7.1 Dawson G, Schroeder C, Dawson PE. Palmitoyl:protein thioesterase (PPT1) inhibitors can act as pharmacological chaperones in infantile Batten disease. Biochem Biophys Res Commun. 2010 Apr 23;395(1):66-9. doi:, 10.1016/j.bbrc.2010.03.137. Epub 2010 Mar 25. PMID:20346914 doi:http://dx.doi.org/10.1016/j.bbrc.2010.03.137
- ↑ Vines DJ, Warburton MJ. Classical late infantile neuronal ceroid lipofuscinosis fibroblasts are deficient in lysosomal tripeptidyl peptidase I. FEBS Lett. 1999 Jan 25;443(2):131-5. PMID:9989590
- ↑ Mitchison HM, Taschner PE, Kremmidiotis G, Callen DF, Doggett NA, Lerner TJ, Janes RB, Wallace BA, Munroe PB, O'Rawe AM, Gardiner RM, Mole SE. Structure of the CLN3 gene and predicted structure, location and function of CLN3 protein. Neuropediatrics. 1997 Feb;28(1):12-4. PMID:9151311 doi:http://dx.doi.org/10.1055/s-2007-973656
Similar Proteopedia Pages
Category:Palmitoyl_protein_thioesterase
External Resources
Gauche Effect Wikipedia page
Palmitic acid Wikipedia page
Infantile Neuronal Ceroid Lipofuscinosis Wikipedia page
PMSF Wikipedia page
Protein Chaperones Wikipedia page
Endoplasmic reticulum Wikipedia page
Palmitoylation Wikipedia page
Page on Late Infantile neuronal Ceroid Lipofuscinosis
Page on Juvenile neuronal Ceroid Lipofuscinosis
Student Contributors
Andrew Bartels
Nicole Green
Kelly Maddalone
Ryan Mughmaw
Audrey Wright
Proteopedia Page Contributors and Editors (what is this?)
R. Jeremy Johnson, Karsten Theis, Alexander Berchansky, Angel Herraez, Michal Harel