We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 123
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load= size=450 side='right' scene='36/365380/4dki_cartoon/ | + | <StructureSection load= size=450 side='right' scene='36/365380/4dki_cartoon/28'> |
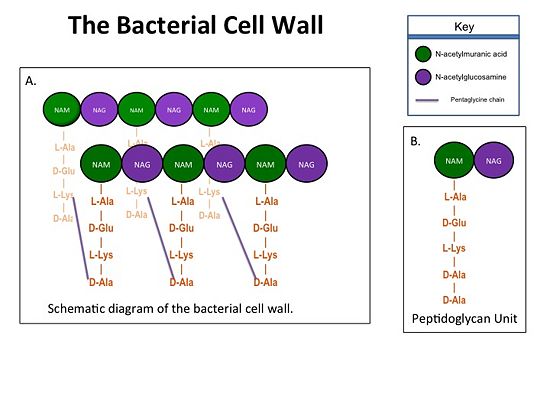
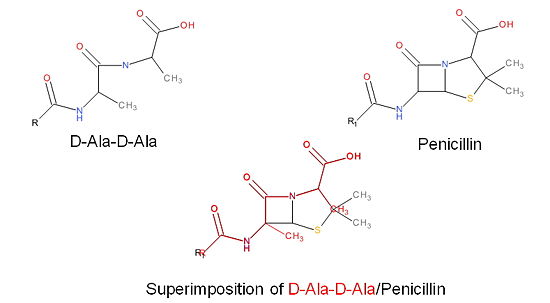
Transpeptidase (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. Beta-lactam (β-lactam) antibiotics, which | Transpeptidase (TP), also known as penicillin-binding proteins (PBP), catalyze the cross-linking of peptidoglycan polymers during bacterial cell wall synthesis. Beta-lactam (β-lactam) antibiotics, which | ||
| Line 13: | Line 13: | ||
== Structure of a Resistant Transpeptidase == | == Structure of a Resistant Transpeptidase == | ||
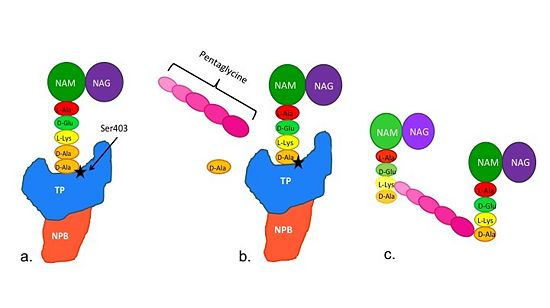
| - | Methicillin resistant Staphylococcus aureus (MRSA) is resistant to all β-lactams because it acquires an alternative PBP, PBP2a, that is not bound or inhibited by any β-lactams. PBP2a is composed of two domains: a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/ | + | Methicillin resistant Staphylococcus aureus (MRSA) is resistant to all β-lactams because it acquires an alternative PBP, PBP2a, that is not bound or inhibited by any β-lactams. PBP2a is composed of two domains: a <font color='orange'><b>non-penicillin binding domain </b><scene name='36/365380/4dki_cartoon/29'>(NPB) </scene></font> and a <font color='dodgerblue'><b>transpeptidase <scene name='36/365380/4dki_cartoon/30'>(TP)</scene> binding domain </b></font>. The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain “sits” in the periplasm with its active site facing the inner surface of the cell wall. The active site contains <scene name='36/365380/Ser403/22'>a serine residue at position 403 (ser403)</scene> which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. |
| Line 42: | Line 42: | ||
MRSA becomes resistant to β-lactams by acquiring an alternative PBP, PBP2a, that is | MRSA becomes resistant to β-lactams by acquiring an alternative PBP, PBP2a, that is | ||
| - | neither bound nor inhibited by β-lactams. Recently, two cephalosporins – <scene name='36/365380/Ceftobiprole/ | + | neither bound nor inhibited by β-lactams. Recently, two cephalosporins – <scene name='36/365380/Ceftobiprole/28'>ceftobiprole</scene> and ceftaroline – that have anti-MRSA activity have been developed. Ceftobiprole is able to |
| - | inhibit PBP2a because additional chemical groups at the <scene name='36/365380/Ceftobiprole/ | + | inhibit PBP2a because additional chemical groups at the <scene name='36/365380/Ceftobiprole/29'>R2</scene> position of the cephalosporin backbone are able to interact with additional amino acid residues in PBP2a; specifically |
| - | <scene name='36/365380/Ceftobiprole/ | + | <scene name='36/365380/Ceftobiprole/30'>Tyr446 and Met641</scene>. As a result of its tighter binding to PBP2a, ceftobiprole is able to more |
efficiently react with the serine active site residue and therefore inhibit the activity of | efficiently react with the serine active site residue and therefore inhibit the activity of | ||
PBP2a. | PBP2a. | ||
| Line 50: | Line 50: | ||
== PBP2a and Ceftaroline == | == PBP2a and Ceftaroline == | ||
| - | <scene name='36/365380/3zfz_1/ | + | <scene name='36/365380/3zfz_1/19'>PBP2a in complex with Ceftaroline</scene> (PDB:3ZFZ) |
| - | In addition to TP domain of PBP2a, there is an allosteric domain,highlighted orange, in which the distance between <scene name='36/365380/3zfz_1/ | + | In addition to TP domain of PBP2a, there is an allosteric domain,highlighted orange, in which the distance between <scene name='36/365380/3zfz_1/20'>the active site and the allosteric site</scene> is 60Å. |
| - | Allosteric site serves as a binding site for the substrate <scene name='36/365380/3zfz_1/7'>peptidoglycan</scene>. When the substrate binds to the <scene name='36/365380/3zfz_1/ | + | Allosteric site serves as a binding site for the substrate <scene name='36/365380/3zfz_1/7'>peptidoglycan</scene>. When the substrate binds to the <scene name='36/365380/3zfz_1/21'>allosteric site</scene> (Tyr105, Asn146, Asp295, Tyr297), a conformational change occurs at the active site, opening it and allowing catalytic action to occur. |
| - | The medicine, <scene name='36/365380/3zfz_1/ | + | The medicine, ceftaroline, mimics the substrate at the allosteric site opening the active site, allowing <scene name='36/365380/3zfz_1/22'>ceftaroline</scene> to <scene name='36/365380/3zfz_1/23'>enter and bind noncovalently</scene>. |
Revision as of 21:26, 5 May 2014
| |||||||||||