We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 126
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==='''Background Information'''=== | ==='''Background Information'''=== | ||
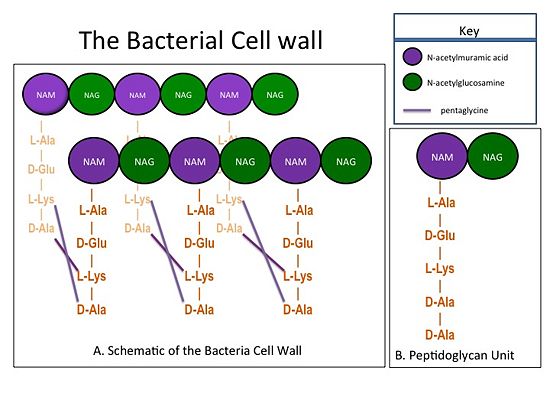
| - | The bacterial cell wall is composed of sheets of peptidoglycan cross-linked together to form a highly polymeric "mesh" that helps maintain the structural strength of the cell (Figure 1). A peptidoglycan sheet consists of alternating residues of <font color='purple'> '''N-acetylmuramic acid (NAM)''' </font> and <font color='green'> '''N-acetylglucosamine (NAG)''' </font> linked together by β-(1,4)- glycosidic bonds. In ''Staphylococcus aureus'' (S. aureus), the NAM residues are coupled to a (D-Ala) residues. The sheets of peptidoglycan are cross-linked together with pentaglycine chains. The cross-linking of adjacent peptidoglycan sheets is catalyzed by transpeptidases (TP). Beta-Lactam antibiotics, such as penicillin and the anti-MRSA cephlosporins, ceftobiprole and ceftaroline, stop the production of the cell wall, and so kill bacteria, by irreversibly inhibiting TPs. Therefore, TPs are also called penicillin-binding proteins.[[Image:CellWall.jpg|thumb|alt= Alt text| Figure 1.(A) This moiety is polymerized to form sheets of peptidoglycan. Adjacent sheets of peptidoglycan are cross-linked together by pentaglycine "bridges" to form a polymeric "mesh" that is essential for the structural integrity of the bacterial cell (B) The cell wall is composed of repeating units of a NAM/NAG disaccharide and peptide moiety; ''i.e.'', peptidoglycan |550px]] | + | The bacterial cell wall is composed of sheets of peptidoglycan cross-linked together to form a highly polymeric "mesh" that helps maintain the structural strength of the cell (Figure 1). A peptidoglycan sheet consists of alternating residues of <font color='purple'> '''N-acetylmuramic acid (NAM)''' </font> and <font color='green'> '''N-acetylglucosamine (NAG)''' </font> linked together by β-(1,4)- glycosidic bonds. In ''Staphylococcus aureus'' (S. aureus), the NAM residues are coupled to a (D-Ala) residues. The sheets of peptidoglycan are cross-linked together with pentaglycine chains. The cross-linking of adjacent peptidoglycan sheets is catalyzed by transpeptidases (TP). Beta-Lactam antibiotics, such as penicillin and the anti-MRSA cephlosporins, ceftobiprole and ceftaroline, stop the production of the cell wall, and so kill bacteria, by irreversibly inhibiting TPs. Therefore, TPs are also called penicillin-binding proteins.[[Image:CellWall.jpg|thumb|alt= Alt text| Figure 1.(A) This moiety is polymerized to form sheets of peptidoglycan. Adjacent sheets of peptidoglycan are cross-linked together by pentaglycine "bridges" to form a polymeric "mesh" that is essential for the structural integrity of the bacterial cell. (B) The cell wall is composed of repeating units of a NAM/NAG disaccharide and peptide moiety; ''i.e.'', peptidoglycan. |550px]] |
| Line 11: | Line 11: | ||
==='''Mechanism of action of Beta-Lactam Antibiotics'''=== | ==='''Mechanism of action of Beta-Lactam Antibiotics'''=== | ||
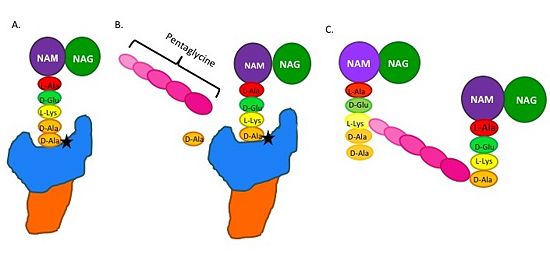
| - | The beta-lactam antibiotics irreversibly bind to and inhibit TPs. This results in the disruption of peptidoglycan synthesis and ultimately cell growth. Specifically, beta-lactams, such as penicillin and the anti-MRSA cephlasporins, ceftobiprole and ceftaroline, are molecular mimics of the peptidoglycan D-Ala-D-Ala moiety; the normal TP substrate (Figure 2; Tipper and Strominger, 1965). Therefore, they "trick" the TP active site serine residue to react with them, resulting in the irreversible inhibition of TP activity and of cell wall synthesis.[[Image: MechanismofPBP.jpg|thumb|alt= Alt text|Figure 2. Schematic showing Catalytic Mechanism of PBP2a (A) the peptidoglycan |550px]] | + | The beta-lactam antibiotics irreversibly bind to and inhibit TPs. This results in the disruption of peptidoglycan synthesis and ultimately cell growth. Specifically, beta-lactams, such as penicillin and the anti-MRSA cephlasporins, ceftobiprole and ceftaroline, are molecular mimics of the peptidoglycan D-Ala-D-Ala moiety; the normal TP substrate (Figure 2; Tipper and Strominger, 1965). Therefore, they "trick" the TP active site serine residue to react with them, resulting in the irreversible inhibition of TP activity and of cell wall synthesis.[[Image: MechanismofPBP.jpg|thumb|alt= Alt text|Figure 2.Schematic showing Catalytic Mechanism of PBP2a (A) The peptidoglycan D-Ala D-Ala moiety enters the TP active site, which is in the TP domain (blue) (B) The active site serine residue (star) reacts with and breaks the peptide bond between the D-Ala residues. The terminal D-Ala residue exits the active site. The remaining D-Ala residue is covalently bound to the active site serine residue, and therefore, to TP. The incoming pentaglycine chain reacts with the bound D-Ala residue and is cross-linked to the D-Ala residue. (D) This results in cross-linking between adjacent peptidoglycan "sheets" and regeneration of the active site serine residue so it can catalyze another cross-linking reaction. |550px]] |
==='''MRSA, PBP2a, and anti-MRSA Cephalosporins'''=== | ==='''MRSA, PBP2a, and anti-MRSA Cephalosporins'''=== | ||
Revision as of 18:20, 31 July 2014
| |||||||||||