Krebs cycle step 2
From Proteopedia
m (Sandbox Oldenburg05 moved to Krebs cycle step 2) |
|||
| Line 1: | Line 1: | ||
| - | <h2>Second Step of the krebs Cycle: Aconitase</h2> | + | <h2>Second Step of the krebs Cycle: [[Aconitase]]</h2> |
[[Image:aconitase.jpg]] | [[Image:aconitase.jpg]] | ||
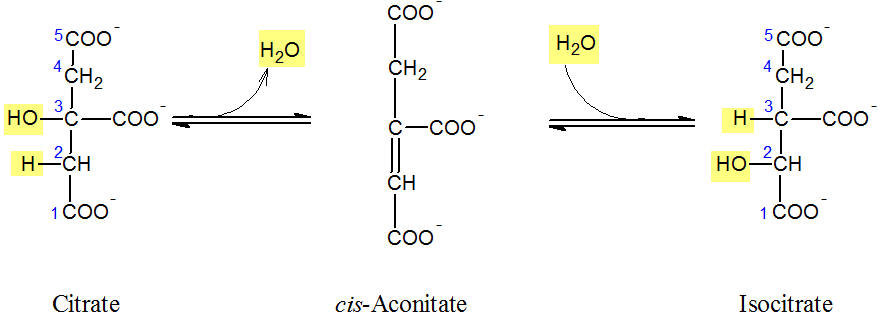
<p>Figure: Reaction of the isomerisation of citrate to isocitrate</p> | <p>Figure: Reaction of the isomerisation of citrate to isocitrate</p> | ||
| - | <p>In the second reaction of the Krebs cycle, the isomerisation of citrate to | + | <p>In the second reaction of the [[Citric Acid Cycle|Krebs cycle]], the isomerisation of citrate to |
isocitrate takes place. As an intermediate, <i>cis</i>-aconitate is formed. Therefore, it is a two-step reaction sequence. In the first step, H<sub>2</sub>O is removed from the cireate molecule (see Figure). Thie dehydration ledas to the intermediate, <i>cis</i>-aconitate which is bound to the enzyme. In the | isocitrate takes place. As an intermediate, <i>cis</i>-aconitate is formed. Therefore, it is a two-step reaction sequence. In the first step, H<sub>2</sub>O is removed from the cireate molecule (see Figure). Thie dehydration ledas to the intermediate, <i>cis</i>-aconitate which is bound to the enzyme. In the | ||
second step, <i>cis</i>-aconitate is hydrated again. Therefore, the proton (from C2 to C3) and the OH group | second step, <i>cis</i>-aconitate is hydrated again. Therefore, the proton (from C2 to C3) and the OH group | ||
Revision as of 09:19, 18 December 2019
Second Step of the krebs Cycle: Aconitase
Figure: Reaction of the isomerisation of citrate to isocitrate
In the second reaction of the Krebs cycle, the isomerisation of citrate to isocitrate takes place. As an intermediate, cis-aconitate is formed. Therefore, it is a two-step reaction sequence. In the first step, H2O is removed from the cireate molecule (see Figure). Thie dehydration ledas to the intermediate, cis-aconitate which is bound to the enzyme. In the second step, cis-aconitate is hydrated again. Therefore, the proton (from C2 to C3) and the OH group (from C3 to C2) swap places. A tretiary aclohol is transformed into a secondary alcohol.
This conversion is of utmost importance for the subsequent decarboxylation, because a tertiary alcohol (the citrate) can not be directly oxidized to a keto carboxylic acid.