We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
JMS/sandbox22
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
'''Molecular Tour:''' | '''Molecular Tour:''' | ||
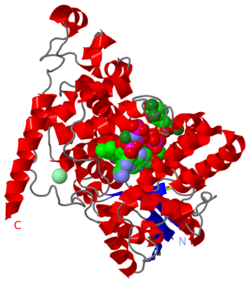
| - | The cryptochrome protein obsorbs a single phton of blue light of 2.7 eV which | + | The cryptochrome protein obsorbs a single phton of blue light of 2.7 eV which excites an <scene name='58/585079/1u3d_magnet/2'>FAD ligand in its interior</scene>. FAD is protonated by a <scene name='58/585079/1u3d_magnet/15'>nearby aspartic amino acid</scene>, and the electron hole is filled through a series of electron transfers - a chain reaction invovling three tryptophan amino acids which form a line extending from FAD in the interior to the surface of the protein. |
Klaus Schulten of the UIUC and Illia Solov'yov, now at the University of Southern Denmark, hypothesize that the FAD factor and just several residues of a crytochrome protein is all it takes to register the magnetic field of the earth. The <scene name='58/585079/1u3d_magnet/2'>"magnetic core"</scene> they describe involves the <scene name='58/585079/1u3d_magnet/15'>FAD factor, three tryptophan residues, as well as the aspartic residues which neighbor the FAD factor</scene>. When light in the blue range hits the FAD factor it becomes excited, with the excitement diffused over its <scene name='58/585079/1u3d_magnet/8'>aromatic ring system</scene> (the atoms involved in resonance are shown with halos). Then, one of the <scene name='58/585079/1u3d_magnet/16'>three neighboring aspartic acid residues</scene> donates a hydrogen proton from its hydroxyl group (the proximate ones shown with halos). The FAD factor then receives an electron from the neighboring tryptophan, from the tryptophan's nitrogen atom (shown in halo). The proton and electron that FAD received are attached to one of the nitrogen atoms on its ring (shown with a halo). Next, this tryptophan received an electron from its <scene name='58/585079/1u3d_magnet/17'>neighboring tryptophan</scene>, and then the second tryptophan received an electron from its neighbor, a third tryptophan. Finally, the third tryptophan loses a proton to a neighboring element. ''At this stage, the magnetic core contains an entangled pair of free radicals.'' The FAD factor contains a <scene name='58/585079/1u3d_magnet/18'>free radical on the adjacent carbon atom</scene> (shown with a halo), as does the third tryptophan residue on its donating nitrogen atom(shown with a halo).<br> | Klaus Schulten of the UIUC and Illia Solov'yov, now at the University of Southern Denmark, hypothesize that the FAD factor and just several residues of a crytochrome protein is all it takes to register the magnetic field of the earth. The <scene name='58/585079/1u3d_magnet/2'>"magnetic core"</scene> they describe involves the <scene name='58/585079/1u3d_magnet/15'>FAD factor, three tryptophan residues, as well as the aspartic residues which neighbor the FAD factor</scene>. When light in the blue range hits the FAD factor it becomes excited, with the excitement diffused over its <scene name='58/585079/1u3d_magnet/8'>aromatic ring system</scene> (the atoms involved in resonance are shown with halos). Then, one of the <scene name='58/585079/1u3d_magnet/16'>three neighboring aspartic acid residues</scene> donates a hydrogen proton from its hydroxyl group (the proximate ones shown with halos). The FAD factor then receives an electron from the neighboring tryptophan, from the tryptophan's nitrogen atom (shown in halo). The proton and electron that FAD received are attached to one of the nitrogen atoms on its ring (shown with a halo). Next, this tryptophan received an electron from its <scene name='58/585079/1u3d_magnet/17'>neighboring tryptophan</scene>, and then the second tryptophan received an electron from its neighbor, a third tryptophan. Finally, the third tryptophan loses a proton to a neighboring element. ''At this stage, the magnetic core contains an entangled pair of free radicals.'' The FAD factor contains a <scene name='58/585079/1u3d_magnet/18'>free radical on the adjacent carbon atom</scene> (shown with a halo), as does the third tryptophan residue on its donating nitrogen atom(shown with a halo).<br> | ||
Revision as of 18:46, 4 August 2014
| |||||||||||
References:
- Cryptochrome and Magnetic Sensing, Theoretical and Computational Biophysics Group at the University of Illinois at Urbana-Champaign