This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
1db1
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1db1 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1db1 OCA], [http://www.ebi.ac.uk/pdbsum/1db1 PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1db1 RCSB]</span> | ||
}} | }} | ||
| Line 14: | Line 17: | ||
==Overview== | ==Overview== | ||
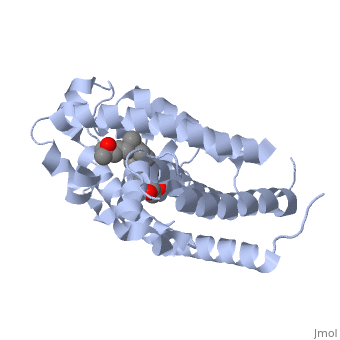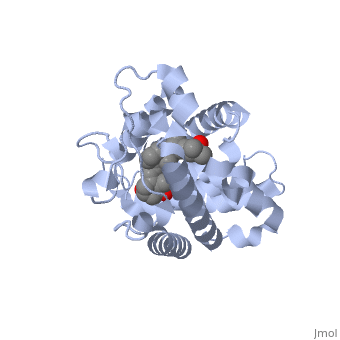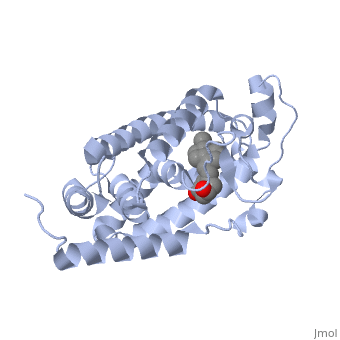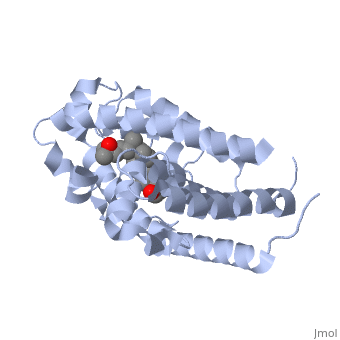
The action of 1 alpha, 25-dihydroxyvitamin D3 is mediated by its nuclear receptor (VDR), a ligand-dependent transcription regulator. We report the 1.8 A resolution crystal structure of the complex between a VDR ligand-binding domain (LBD) construct lacking the highly variable VDR-specific insertion domain and vitamin D. The construct exhibits the same binding affinity for vitamin D and transactivation ability as the wild-type protein, showing that the N-terminal part of the LBD is essential for its structural and functional integrity while the large insertion peptide is dispensable. The structure reveals the active conformation of the bound ligand and allows understanding of the different binding properties of some synthetic analogs. | The action of 1 alpha, 25-dihydroxyvitamin D3 is mediated by its nuclear receptor (VDR), a ligand-dependent transcription regulator. We report the 1.8 A resolution crystal structure of the complex between a VDR ligand-binding domain (LBD) construct lacking the highly variable VDR-specific insertion domain and vitamin D. The construct exhibits the same binding affinity for vitamin D and transactivation ability as the wild-type protein, showing that the N-terminal part of the LBD is essential for its structural and functional integrity while the large insertion peptide is dispensable. The structure reveals the active conformation of the bound ligand and allows understanding of the different binding properties of some synthetic analogs. | ||
| - | |||
| - | ==Disease== | ||
| - | Known diseases associated with this structure: Osteoporosis, involutional, 166710 (1) OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=601769 601769]], Rickets, vitamin D-resistant, type IIA OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=601769 601769]] | ||
==About this Structure== | ==About this Structure== | ||
| Line 30: | Line 30: | ||
[[Category: Rochel, N.]] | [[Category: Rochel, N.]] | ||
[[Category: Wurtz, J M.]] | [[Category: Wurtz, J M.]] | ||
| - | [[Category: VDX]] | ||
[[Category: complex]] | [[Category: complex]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 19:38:10 2008'' |
Revision as of 16:38, 30 March 2008
| |||||||
| , resolution 1.8Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
CRYSTAL STRUCTURE OF THE NUCLEAR RECEPTOR FOR VITAMIN D COMPLEXED TO VITAMIN D
Overview
The action of 1 alpha, 25-dihydroxyvitamin D3 is mediated by its nuclear receptor (VDR), a ligand-dependent transcription regulator. We report the 1.8 A resolution crystal structure of the complex between a VDR ligand-binding domain (LBD) construct lacking the highly variable VDR-specific insertion domain and vitamin D. The construct exhibits the same binding affinity for vitamin D and transactivation ability as the wild-type protein, showing that the N-terminal part of the LBD is essential for its structural and functional integrity while the large insertion peptide is dispensable. The structure reveals the active conformation of the bound ligand and allows understanding of the different binding properties of some synthetic analogs.
About this Structure
1DB1 is a Single protein structure of sequence from Homo sapiens. Full crystallographic information is available from OCA.
Reference
The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand., Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D, Mol Cell. 2000 Jan;5(1):173-9. PMID:10678179
Page seeded by OCA on Sun Mar 30 19:38:10 2008