We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
2fmp
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Structural highlights == | == Structural highlights == | ||
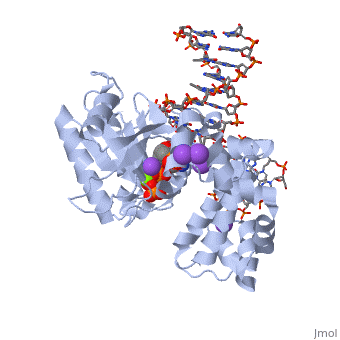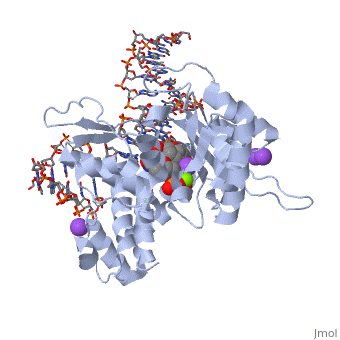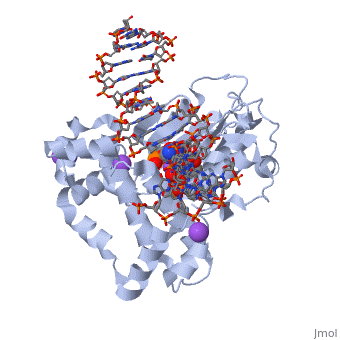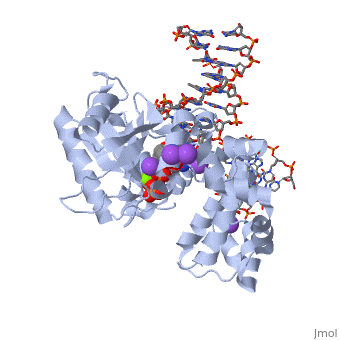
<table><tr><td colspan='2'>[[2fmp]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2FMP OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2FMP FirstGlance]. <br> | <table><tr><td colspan='2'>[[2fmp]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2FMP OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2FMP FirstGlance]. <br> | ||
| - | </td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=DCT:2,3-DIDEOXYCYTIDINE+5-TRIPHOSPHATE'>DCT</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NA:SODIUM+ION'>NA</scene>< | + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=DCT:2,3-DIDEOXYCYTIDINE+5-TRIPHOSPHATE'>DCT</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NA:SODIUM+ION'>NA</scene></td></tr> |
| - | <tr><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=DOC:2,3-DIDEOXYCYTIDINE-5-MONOPHOSPHATE'>DOC</scene></td></tr> | + | <tr id='NonStdRes'><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=DOC:2,3-DIDEOXYCYTIDINE-5-MONOPHOSPHATE'>DOC</scene></td></tr> |
| - | <tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2fmq|2fmq]], [[2fms|2fms]]</td></tr> | + | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2fmq|2fmq]], [[2fms|2fms]]</td></tr> |
| - | <tr><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">POLB ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> | + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">POLB ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 Homo sapiens])</td></tr> |
| - | <tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2fmp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2fmp OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2fmp RCSB], [http://www.ebi.ac.uk/pdbsum/2fmp PDBsum]</span></td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2fmp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2fmp OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2fmp RCSB], [http://www.ebi.ac.uk/pdbsum/2fmp PDBsum]</span></td></tr> |
| - | <table> | + | </table> |
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/DPOLB_HUMAN DPOLB_HUMAN]] Repair polymerase that plays a key role in base-excision repair. Has 5'-deoxyribose-5-phosphate lyase (dRP lyase) activity that removes the 5' sugar phosphate and also acts as a DNA polymerase that adds one nucleotide to the 3' end of the arising single-nucleotide gap. Conducts 'gap-filling' DNA synthesis in a stepwise distributive fashion rather than in a processive fashion as for other DNA polymerases.<ref>PMID:9207062</ref> <ref>PMID:9572863</ref> <ref>PMID:11805079</ref> <ref>PMID:21362556</ref> | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 35: | Line 37: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| - | [[Category: Batra, V K | + | [[Category: Batra, V K]] |
| - | [[Category: Beard, W A | + | [[Category: Beard, W A]] |
| - | [[Category: Krahn, J M | + | [[Category: Krahn, J M]] |
| - | [[Category: Pedersen, L C | + | [[Category: Pedersen, L C]] |
| - | [[Category: Shock, D D | + | [[Category: Shock, D D]] |
| - | [[Category: Wilson, S H | + | [[Category: Wilson, S H]] |
[[Category: Nucleotidyl transferase]] | [[Category: Nucleotidyl transferase]] | ||
[[Category: Transferase-dna complex]] | [[Category: Transferase-dna complex]] | ||
Revision as of 17:12, 25 December 2014
DNA Polymerase beta with a terminated gapped DNA substrate and ddCTP with sodium in the catalytic site
| |||||||||||