1hew
From Proteopedia
| Line 4: | Line 4: | ||
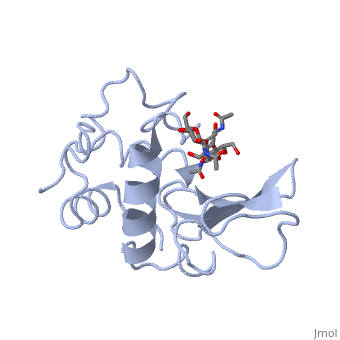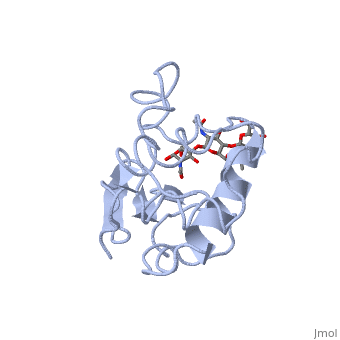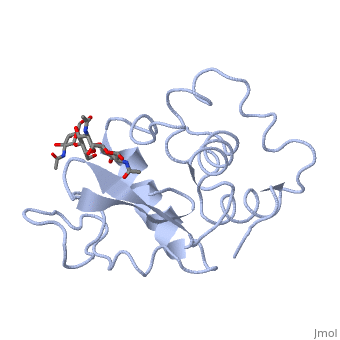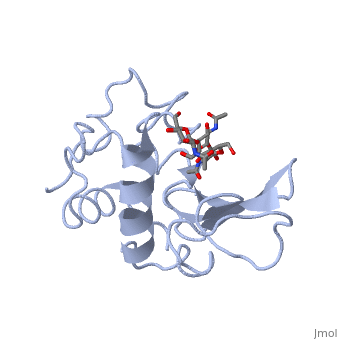
|PDB= 1hew |SIZE=350|CAPTION= <scene name='initialview01'>1hew</scene>, resolution 1.75Å | |PDB= 1hew |SIZE=350|CAPTION= <scene name='initialview01'>1hew</scene>, resolution 1.75Å | ||
|SITE= | |SITE= | ||
| - | |LIGAND= | + | |LIGAND= <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene> |
| - | |ACTIVITY= [http://en.wikipedia.org/wiki/Lysozyme Lysozyme], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.2.1.17 3.2.1.17] | + | |ACTIVITY= <span class='plainlinks'>[http://en.wikipedia.org/wiki/Lysozyme Lysozyme], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.2.1.17 3.2.1.17] </span> |
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1hew FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1hew OCA], [http://www.ebi.ac.uk/pdbsum/1hew PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1hew RCSB]</span> | ||
}} | }} | ||
| Line 27: | Line 30: | ||
[[Category: hydrolase(o-glycosyl)]] | [[Category: hydrolase(o-glycosyl)]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 21:03:42 2008'' |
Revision as of 18:03, 30 March 2008
| |||||||
| , resolution 1.75Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Activity: | Lysozyme, with EC number 3.2.1.17 | ||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
REFINEMENT OF AN ENZYME COMPLEX WITH INHIBITOR BOUND AT PARTIAL OCCUPANCY. HEN EGG-WHITE LYSOZYME AND TRI-N-ACETYLCHITOTRIOSE AT 1.75 ANGSTROMS RESOLUTION
Overview
The structure of the tri-N-acetylchitotriose inhibitor complex of hen egg-white lysozyme has been refined at 1.75 A resolution, using data collected from a complex crystal with ligand bound at less than full occupancy. To determine the exact value of the inhibitor occupancy, a model comprising unliganded and sugar-bound protein molecules was generated and refined against the 1.75 A data, using a modified version of the Hendrickson & Konnert least-squares procedure. The crystallographic R-factor for the model was found to fall to a minimum at 55% bound sugar. Conventional refinement assuming unit occupancy was found to yield incorrect thermal and positional parameters. Application of the same refinement procedures to an earlier 2.0 A data set, collected independently on different complex crystals by Blake et al. gave less consistent results than the 1.75 A refinement. From an analysis of the high resolution structure a detailed picture of the protein-carbohydrate interactions in the non-productive complex has emerged, together with the conformation and mobility changes that accompany ligand binding. The specificity of interaction between the protein and inhibitor, bound in subsites A to C of the active site, is seen to be generated primarily by an extensive network of hydrogen bonds, both to the protein itself and to bound solvent molecules. The latter also play an important role in maintaining the structural integrity of the active site cleft in the apo-protein.
About this Structure
1HEW is a Single protein structure of sequence from [1]. Full crystallographic information is available from OCA.
Reference
Refinement of an enzyme complex with inhibitor bound at partial occupancy. Hen egg-white lysozyme and tri-N-acetylchitotriose at 1.75 A resolution., Cheetham JC, Artymiuk PJ, Phillips DC, J Mol Biol. 1992 Apr 5;224(3):613-28. PMID:1569548
Page seeded by OCA on Sun Mar 30 21:03:42 2008