Journal:JBIC:28
From Proteopedia
(Difference between revisions)

| Line 5: | Line 5: | ||
<b>Molecular Tour</b><br> | <b>Molecular Tour</b><br> | ||
<scene name='60/602702/Cv/2'>[NiFe]-hydrogenases</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">small subunit is in cyan</span> and <font color='magenta'><b>large subunit is in magenta</b></font>) attract much interest because of their ability to cleave or produce the chemical bond in the simplest of energy carriers, molecular hydrogen. A major problem for potential biotechnological applications of most of these enzymes is their inactivation by molecular oxygen. In order to study the chemical processes responsible for the formation of unready, difficult to activate oxidized states of such oxygen-sensitive [NiFe]-hydrogenases, this paper describes a detailed spectroscopic and structural analysis of enzyme mutants with special properties. So far, progress in the fundamental understanding of the reactions with molecular oxygen was limited by problems of structural heterogeneity resulting from the very rich redox chemistry of the active site of these enzymes. In addition, the oxidized unready (difficult to activate) states were difficult to characterize by X-ray crystallographic methods because of the reducing effects of X-ray induced photoelectrons. This study shows how the oxygen-sensitive [NiFe]-hydrogenase of the sulfate-reducing bacterium ''Desulfovibrio fructosovorans'' reacts, under air, with oxygen and metabolic sulfur, forming oxidized thiolate ligands. In one mutant no reaction with sulfur takes place and a pure unready <scene name='60/602702/Cv/5'>Ni-A state is obtained, containing Ni(III) with both sulfenate and hydroxide ligands</scene>. | <scene name='60/602702/Cv/2'>[NiFe]-hydrogenases</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">small subunit is in cyan</span> and <font color='magenta'><b>large subunit is in magenta</b></font>) attract much interest because of their ability to cleave or produce the chemical bond in the simplest of energy carriers, molecular hydrogen. A major problem for potential biotechnological applications of most of these enzymes is their inactivation by molecular oxygen. In order to study the chemical processes responsible for the formation of unready, difficult to activate oxidized states of such oxygen-sensitive [NiFe]-hydrogenases, this paper describes a detailed spectroscopic and structural analysis of enzyme mutants with special properties. So far, progress in the fundamental understanding of the reactions with molecular oxygen was limited by problems of structural heterogeneity resulting from the very rich redox chemistry of the active site of these enzymes. In addition, the oxidized unready (difficult to activate) states were difficult to characterize by X-ray crystallographic methods because of the reducing effects of X-ray induced photoelectrons. This study shows how the oxygen-sensitive [NiFe]-hydrogenase of the sulfate-reducing bacterium ''Desulfovibrio fructosovorans'' reacts, under air, with oxygen and metabolic sulfur, forming oxidized thiolate ligands. In one mutant no reaction with sulfur takes place and a pure unready <scene name='60/602702/Cv/5'>Ni-A state is obtained, containing Ni(III) with both sulfenate and hydroxide ligands</scene>. | ||
| - | The atoms are colored according CPK Color Scheme{{Template:ColorKey_Element_C}} | + | The atoms are colored according CPK Color Scheme: {{Template:ColorKey_Element_C}} |
{{Template:ColorKey_Element_O}} | {{Template:ColorKey_Element_O}} | ||
{{Template:ColorKey_Element_N}} | {{Template:ColorKey_Element_N}} | ||
{{Template:ColorKey_Element_S}} | {{Template:ColorKey_Element_S}} | ||
{{Template:ColorKey_Element_Fe}} | {{Template:ColorKey_Element_Fe}} | ||
| - | + | <font color='green'><b>Ni</b></font>, except for <font color='magenta'><b>carbon atoms of the protein, which are in magenta</b></font> | |
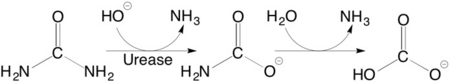
[[Image:Scheme_1.png|left|450px|thumb|]] | [[Image:Scheme_1.png|left|450px|thumb|]] | ||
{{Clear}} | {{Clear}} | ||
Revision as of 10:18, 19 October 2014
| |||||||||||
- ↑ REF
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.