We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
P53-DNA Recognition
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
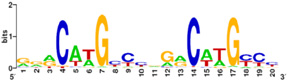
[[Image:p53-motif.jpg|thumb|right|300px|Figure 5: p53 binding site motif with G/C base pairs most conserved. PLoS has provided permission for usage of this figure<ref>Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: cell cycle versus apoptosis. PLoS Genet. 2007 Jul;3(7):e127. [http://www.ncbi.nlm.nih.gov/pubmed/17677004 PMID:17677004].</ref>.]] | [[Image:p53-motif.jpg|thumb|right|300px|Figure 5: p53 binding site motif with G/C base pairs most conserved. PLoS has provided permission for usage of this figure<ref>Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: cell cycle versus apoptosis. PLoS Genet. 2007 Jul;3(7):e127. [http://www.ncbi.nlm.nih.gov/pubmed/17677004 PMID:17677004].</ref>.]] | ||
| - | + | {{Clear}} | |
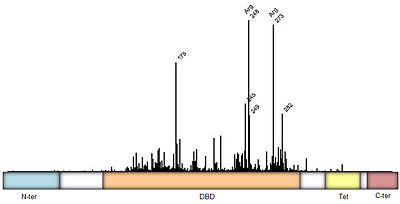
Protein side chains and base pairs form direct contacts in the major groove. Among which, the <scene name='Sandbox_Reserved_170/Arg280_contact/5'>contact between Arg280 and the guanine of the core element</scene> contributes most to binding specificity. This highly specific readout is due to the <scene oldname='Sandbox_Reserved_170/Arg280_contact/4' name='P53-DNA_Recognition/P53_arg280_contact/1'>bidentate hydrogen bond formed between Arg280 and guanine</scene>. As a result of this '''base readout''' the G/C base pairs in the CWWG core elements are the most conserved positions in p53 response elements ('''Figure 5'''). | Protein side chains and base pairs form direct contacts in the major groove. Among which, the <scene name='Sandbox_Reserved_170/Arg280_contact/5'>contact between Arg280 and the guanine of the core element</scene> contributes most to binding specificity. This highly specific readout is due to the <scene oldname='Sandbox_Reserved_170/Arg280_contact/4' name='P53-DNA_Recognition/P53_arg280_contact/1'>bidentate hydrogen bond formed between Arg280 and guanine</scene>. As a result of this '''base readout''' the G/C base pairs in the CWWG core elements are the most conserved positions in p53 response elements ('''Figure 5'''). | ||
| Line 45: | Line 45: | ||
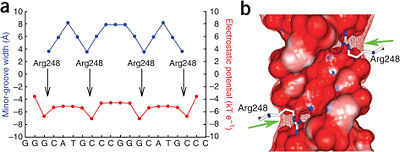
[[Image:Kitayner-etal-Figure7.jpg|thumb|right|400px|Figure 6: DNA shape readout of narrow minor groove regions with enhanced electrostatic potential by Arg248. Nature Publishing Group has provided permission for usage of this figure<ref name='kitayner'/>.]] | [[Image:Kitayner-etal-Figure7.jpg|thumb|right|400px|Figure 6: DNA shape readout of narrow minor groove regions with enhanced electrostatic potential by Arg248. Nature Publishing Group has provided permission for usage of this figure<ref name='kitayner'/>.]] | ||
| - | + | {{Clear}} | |
===Minor Groove Shape Readout=== | ===Minor Groove Shape Readout=== | ||
| Line 58: | Line 58: | ||
==Tetramerization Domain== | ==Tetramerization Domain== | ||
| - | < | + | <scene name='Sandbox_Reserved_170/Tetra/2'>Figure 7: Crystal structure of p53 tetramerization domain</scene>, ([[1c26|PDB ID 1C26]]) |
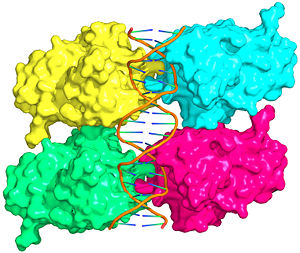
Aside from the DBD, the only other domain for which structural information is available is the ''tetramerization domain'' ['''Figure 7''', <scene name='Sandbox_Reserved_170/Tetra/2'>restore initial scene</scene>], which forms as a dimer of dimers with one alpha helix and one beta strand contributed by each p53 monomer. The tetramerization domain is ''not present'' in the crystal structure of the DBD (Figure 4). | Aside from the DBD, the only other domain for which structural information is available is the ''tetramerization domain'' ['''Figure 7''', <scene name='Sandbox_Reserved_170/Tetra/2'>restore initial scene</scene>], which forms as a dimer of dimers with one alpha helix and one beta strand contributed by each p53 monomer. The tetramerization domain is ''not present'' in the crystal structure of the DBD (Figure 4). | ||
| Line 76: | Line 76: | ||
A more general discussion of structural origins of binding specificity in protein-DNA recognition has been published along with a suggestion for a new '''classification of protein-DNA readout modes''' that goes beyond the historical description of direct and indirect readout<ref name="annualreview">Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS. Origins of specificity in protein-DNA recognition. Annu Rev Biochem. 2010;79:233-69. [http://www.ncbi.nlm.nih.gov/pubmed/20334529 PMID:20334529].</ref>.<br/> | A more general discussion of structural origins of binding specificity in protein-DNA recognition has been published along with a suggestion for a new '''classification of protein-DNA readout modes''' that goes beyond the historical description of direct and indirect readout<ref name="annualreview">Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS. Origins of specificity in protein-DNA recognition. Annu Rev Biochem. 2010;79:233-69. [http://www.ncbi.nlm.nih.gov/pubmed/20334529 PMID:20334529].</ref>.<br/> | ||
| + | </StructureSection> | ||
| + | __NOTOC__ | ||
=Acknowledgements= | =Acknowledgements= | ||
Revision as of 12:48, 2 November 2014
| |||||||||||
Acknowledgements
This Proteopedia page originates from the partnership of the Rohs Laboratory at the University of Southern California with La Cañada High School. This partnership was initiated by Remo Rohs and Patty Compeau in September 2011 as Bioinformatics Institute, which is part of the Institutes of the 21st Century. Advice and technical help by Proteopedia editors Eran Hodis, Eric Martz, Jaime Prilusky, and Joel Sussman is acknowledged.
References
- ↑ 1.0 1.1 1.2 1.3 Kitayner M, Rozenberg H, Rohs R, Suad O, Rabinovich D, Honig B, Shakked Z. Diversity in DNA recognition by p53 revealed by crystal structures with Hoogsteen base pairs. Nat Struct Mol Biol. 2010;17(4):423-9. PMID:20364130.
- ↑ Jeffrey PD, Gorina S, Pavletich NP. Crystal structure of the p53 tetramerization domain. Science 1995;267:1498-502. PMID:7878469.
- ↑ Kitayner M, Rozenberg H, Kessler N, Rabinovich D, Shaulov L, Haran TE, Shakked Z. Structural basis of DNA recognition by p53 tetramers. Mol Cell. 2006 Jun 23;22(6):741-53. PMID:16793544.
- ↑ Horvath MM, Wang X, Resnick MA, Bell DA. Divergent evolution of human p53 binding sites: cell cycle versus apoptosis. PLoS Genet. 2007 Jul;3(7):e127. PMID:17677004.
- ↑ Rohs R, West SM, Sosinsky A, Liu P, Mann RS, Honig B. The role of DNA shape in protein-DNA recognition. Nature. 2009;461(7268):1248-53. PMID:19865164.
- ↑ Aishima J, Gitti RK, Noah JE, Gan HH, Schlick T, Wolberger C. A Hoogsteen base pair embedded in undistorted B-DNA. Nucleic Acids Res. 2002;30(23):5244-52. PMID:12466549.
- ↑ Chen Y, Dey R, Chen L. Crystal structure of the p53 core domain bound to a full consensus site as a self-assembled tetramer. Structure. 2010;18(2):246-56. PMID:20159469.
- ↑ Nikolova EN, Kim E, Wise AA, O'Brien PJ, Andricioaei I, Al-Hashimi HM. Transient Hoogsteen base pairs in canonical duplex DNA. Nature. 2011;470(7335):498-502. PMID:21270796.
- ↑ Rohs R, Jin X, West SM, Joshi R, Honig B, Mann RS. Origins of specificity in protein-DNA recognition. Annu Rev Biochem. 2010;79:233-69. PMID:20334529.
Proteopedia Page Contributors and Editors (what is this?)
Remo Rohs, Eric Martz, Alexander Berchansky, Julia Tam, Sharon Kim, Bailey Holmes, Angel Herraez, Joseph M. Steinberger, Eran Hodis, Masha Karelina, Michal Harel, Ana Carolina Dantas Machado, Jaime Prilusky, Skyler Saleebyan, Joel L. Sussman, Keziah Kim