We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Telomerase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
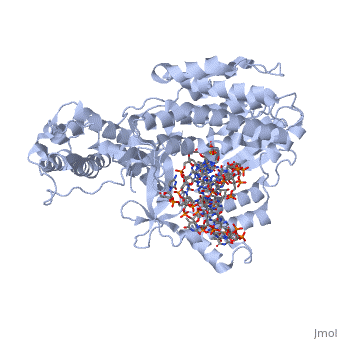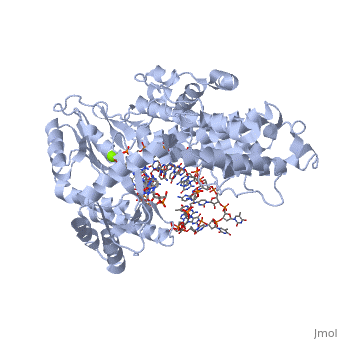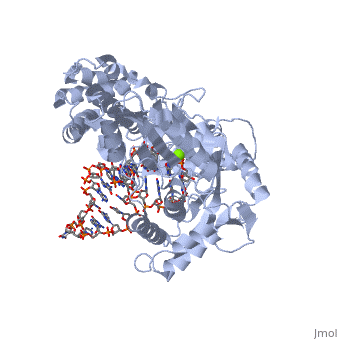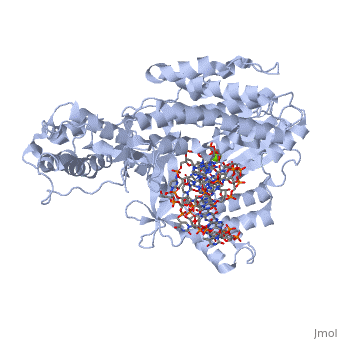
<StructureSection load='3kyl' size='350' side='right' caption='Telomerase: bound to telomeric DNA [[3kyl]]' scene=''> | <StructureSection load='3kyl' size='350' side='right' caption='Telomerase: bound to telomeric DNA [[3kyl]]' scene=''> | ||
== Introduction == | == Introduction == | ||
| - | The telomerase is a complex composed of an RNA primer and protein that adds G-rich repeated DNA sequences to a 3' DNA strand in eukaryotic cells <ref name='complex'>DOI: 10.1146/annurev.bi.61.070192.000553</ref>. This ribonucleoprotein acts as a reverse transcriptase that creates a DNA sequence complementary to its RNA primer in its catalytic subunit, TERT | + | The telomerase is a complex composed of an RNA primer and protein that adds G-rich repeated DNA sequences to a 3' DNA strand in eukaryotic cells <ref name='complex'>DOI: 10.1146/annurev.bi.61.070192.000553</ref>. This ribonucleoprotein acts as a reverse transcriptase that creates a DNA sequence complementary to its RNA primer in its catalytic subunit, TERT <ref name='end'>DOI: 10.1002/anie.201002387</ref>. The sequences attached to the end of DNA is non-coding DNA that functions as protection from mutation and degradation. As DNA replications occurs, the polymerase that copies in the 5' to 3' direction, cannot copy the end of the lagging strand which leaves unpaired bases that may code for a specific gene <ref name='corey'>DOI 10.1016/j.chembiol.2009.12.001</ref>. Multiple unpaired ends can form hydrogen bonds or even swap genetic material. The telomerase fixes this issue and lengthens the strands, because the replication process slowly shortens the ends of chromosomes. This enzyme also plays a role in coordinating cell division. Complementary issues of aging and cancer describe the inactivation or over-activation of telomerase activity <ref name='corey'/>. |
== History == | == History == | ||
| - | In the 1930s, Barbara McClintock was the first to point out that the ends of chromosomes are somewhat different. Over forty years later, Olovnikov (1973) and Watson (1972) indicated an 'end replication problem' in which the lagging strand of DNA cannot be fully copied | + | In the 1930s, Barbara McClintock was the first to point out that the ends of chromosomes are somewhat different. Over forty years later, Olovnikov (1973) and Watson (1972) indicated an 'end replication problem' in which the lagging strand of DNA cannot be fully copied <ref name='end'/>. This concept led to a waterfall of studies and information vitally important to cellular regulation. In 1978, acclaimed chemist and front-runner in telomerase studies, Elizabeth H. Blackburn, discovered the telomere in ''Tetrahymena thermophila''. By 1985, Blackburn and Carol Greider noted the enzymatic activity that created the telomeric sequences, and in 1987, the two women officially identified the telomerase as a ribonucleaoprotein with an essential RNA and and protein component. Elizabeth Blackburn, Carol Greider, and Jack Szostak won the 2009 Noble Prize in Physiology or Medicine for their lifetime work regarding the telomere and telomerase <ref name='corey'/>. |
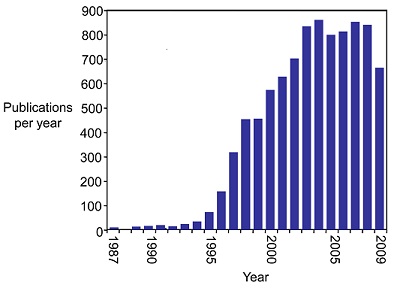
Since 1987, the study of the telomerase has exploded (see figure below)(3) mainly due to its connections to cancer. A multitude of studies in the mid-1990s indicated that many types of cancer cells show increased telomerase activity. By 2001, "telomerase activity had been observed in greater than 90% of patient samples from a wide range of different cancers" <ref name='corey'/>. As a result, strategies to create telomerase inhibitors have been a focus in cancer research. One strategy that has seen success is creating oligonucleotides complementary to the RNA component that acts as a template for telomere formation. As of 2009, a handful of drugs containing these oligonucleotides were clinically approved as cancer treatment, and many more were in pre-clinical trials <ref name='corey'/>. | Since 1987, the study of the telomerase has exploded (see figure below)(3) mainly due to its connections to cancer. A multitude of studies in the mid-1990s indicated that many types of cancer cells show increased telomerase activity. By 2001, "telomerase activity had been observed in greater than 90% of patient samples from a wide range of different cancers" <ref name='corey'/>. As a result, strategies to create telomerase inhibitors have been a focus in cancer research. One strategy that has seen success is creating oligonucleotides complementary to the RNA component that acts as a template for telomere formation. As of 2009, a handful of drugs containing these oligonucleotides were clinically approved as cancer treatment, and many more were in pre-clinical trials <ref name='corey'/>. | ||
| Line 31: | Line 31: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | |||
| - | 2. Blackburn, Elizabeth H. "Telomeres and Telomerase: The Means to the End (Noble Lecture)." Angewandte Chemie International Edition 49 (2010): 7405-421. | ||
| - | |||
</StructureSection> | </StructureSection> | ||
Revision as of 09:24, 6 November 2014
| |||||||||||