1lis
From Proteopedia
| Line 7: | Line 7: | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
| + | |DOMAIN= | ||
| + | |RELATEDENTRY= | ||
| + | |RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1lis FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1lis OCA], [http://www.ebi.ac.uk/pdbsum/1lis PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1lis RCSB]</span> | ||
}} | }} | ||
| Line 28: | Line 31: | ||
[[Category: fertilization protein]] | [[Category: fertilization protein]] | ||
| - | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | + | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Sun Mar 30 22:03:20 2008'' |
Revision as of 19:03, 30 March 2008
| |||||||
| , resolution 1.9Å | |||||||
|---|---|---|---|---|---|---|---|
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
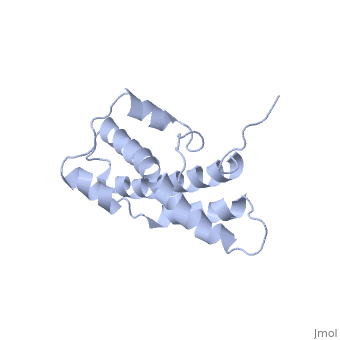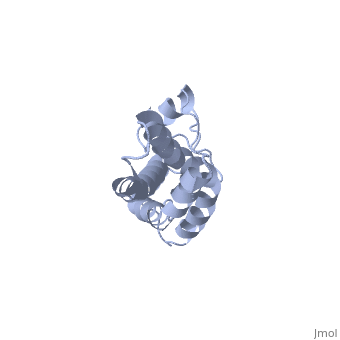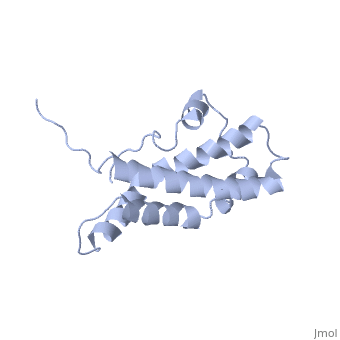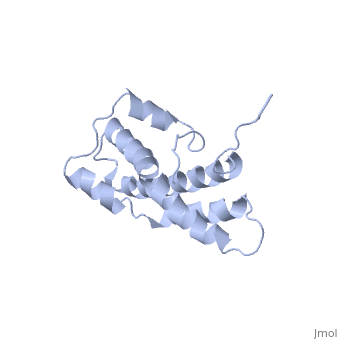
THE CRYSTAL STRUCTURE OF A FERTILIZATION PROTEIN
Overview
Lysin, a protein from abalone sperm, creates a hole in the envelope of the egg, permitting the sperm to pass through the envelope and fuse with the egg. The structure of lysin, refined at 1.9 angstroms resolution, reveals an alpha-helical, amphipathic molecule. The surface of the protein exhibits three features: two tracks of basic residues that span the length of the molecule, a solvent-exposed cluster of aromatic and aliphatic amino acids, and an extended amino-terminal hypervariable domain that is species-specific. The structure suggests possible mechanisms of action.
About this Structure
1LIS is a Single protein structure of sequence from Haliotis rufescens. Full crystallographic information is available from OCA.
Reference
The crystal structure of lysin, a fertilization protein., Shaw A, McRee DE, Vacquier VD, Stout CD, Science. 1993 Dec 17;262(5141):1864-7. PMID:8266073
Page seeded by OCA on Sun Mar 30 22:03:20 2008