Elizabeth Cummings/sandbox 1
From Proteopedia
| Line 1: | Line 1: | ||
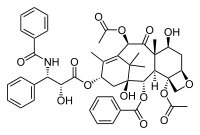
| - | + | [[Image:Paclitaxel.png|frame|Paclitaxel (Taxol, Bristol-Myers Squibb)]] | |
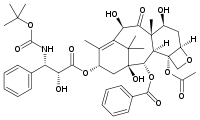
| - | + | [[Image:Docetaxel.png|frame|Docetaxel (Taxotere, Sanofi-aventis)]] | |
| - | + | ||
| - | + | ||
| - | == Function == | ||
| - | + | Paclitaxel is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Paclitaxel, also called Taxol (Bristol-Myers Squibb), is a plant derived anti-cancer agent that was first isolated from the bark of Pacific yew tree, ''Taxus brevifolia'', in 1971. It is a complex diterpenoid with a bulky, fused ring system as well as a number of hydrophobic substituents. Approved by the FDA in 1992, it is currently being used in the treatment of ovarian, breast and lung cancers. In addition, therapies are being developed for treatment of Alzheimer's and post-heart surgery patients. | |
| - | This is a | + | Originally, paclitaxel was produced through the extraction of the drug from the bark of ''Taxus'' trees. This process was unsustainable because nearly 40,000 mature trees were required to meet the demands for the drug each year. A semi-synthetic route was developed and utilized for paclitaxel production using Taxol precursors, which can be extracted from the needles of ''Taxus'' trees. This process is more sustainable because the ''Taxus'' needles can be harvested depending on their seasonal availability without the destruction of the tree, but the process uses harsh, expensive solvents and has a low product yield. In 2004, the production of paclitaxel through plant cell culture was approved by the FDA. This was the first plant cell culture production route approved for the production of a pharmaceutical. Currently, [http://www.bms.com/pages/default.aspx Bristol-Myers Squibb] is producing paclitaxel for pharmaceutical use solely through the sustainable plant cell culture process. |
| - | </ | + | Docetaxel (Taxotere, sanofi-aventis) is a semi-synthetic analog of Taxol that was discovered during the search for a more easily produced taxane anti-cancer agent. The hydroxyl group modification on docetaxel leads to an increase in the lipid solubility of the drug. It was first approved by the FDA in 1996 and is currently used in the treatment of breast, stomach and prostate cancer. Currently, Taxotere is produced from paclitaxel precursors which are extracted from ''Taxus brevifolia'', the readily available Wester Yew. |
| - | == | + | |
| - | < | + | Both Taxol and Taxotere bind to cell microtubules, promoting their assembly into bundles and preventing cell mitosis. This eventually leads to the death of the cells. Although the mechanism of action for both drugs is the same, Taxotere has been found to be twice as potent as Taxol. |
| + | |||
| + | <applet size='[450,338]' frame='true' align='left' | ||
| + | caption='Paclitaxel (also known as Taxol)' /> <scene name='Rohan_Patil/Sandbox1/Taxol/6'>Paclitaxel</scene> | ||
| + | |||
| + | Molecular Playground Banner: "Paclitaxel (Taxol),a plant-derived natural product to treat cancer" | ||
| + | |||
| + | <applet size='[450,338]' frame='true' align='right' | ||
| + | caption='Docetaxel(also known as Taxotere)' /> <scene name='Molecular_Playground/Taxol/Docetaxel/4'>Docetaxel</scene> | ||
| + | |||
| + | Molecular Playground Banner: "Docetaxel (Taxotere), an analaog of the plant derived anti-cancer agent paclitaxel" | ||
| + | |||
| + | <applet size='[450,338]' frame='true' align='left' | ||
| + | caption='Paclitaxel binding to alpha-beta tubulin' /> <scene name='60/609785/Tubulin/2'>Paclitaxel binding to alpha-beta tubulin</scene> | ||
Revision as of 18:48, 4 December 2014
Paclitaxel is one of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Paclitaxel, also called Taxol (Bristol-Myers Squibb), is a plant derived anti-cancer agent that was first isolated from the bark of Pacific yew tree, Taxus brevifolia, in 1971. It is a complex diterpenoid with a bulky, fused ring system as well as a number of hydrophobic substituents. Approved by the FDA in 1992, it is currently being used in the treatment of ovarian, breast and lung cancers. In addition, therapies are being developed for treatment of Alzheimer's and post-heart surgery patients.
Originally, paclitaxel was produced through the extraction of the drug from the bark of Taxus trees. This process was unsustainable because nearly 40,000 mature trees were required to meet the demands for the drug each year. A semi-synthetic route was developed and utilized for paclitaxel production using Taxol precursors, which can be extracted from the needles of Taxus trees. This process is more sustainable because the Taxus needles can be harvested depending on their seasonal availability without the destruction of the tree, but the process uses harsh, expensive solvents and has a low product yield. In 2004, the production of paclitaxel through plant cell culture was approved by the FDA. This was the first plant cell culture production route approved for the production of a pharmaceutical. Currently, Bristol-Myers Squibb is producing paclitaxel for pharmaceutical use solely through the sustainable plant cell culture process.
Docetaxel (Taxotere, sanofi-aventis) is a semi-synthetic analog of Taxol that was discovered during the search for a more easily produced taxane anti-cancer agent. The hydroxyl group modification on docetaxel leads to an increase in the lipid solubility of the drug. It was first approved by the FDA in 1996 and is currently used in the treatment of breast, stomach and prostate cancer. Currently, Taxotere is produced from paclitaxel precursors which are extracted from Taxus brevifolia, the readily available Wester Yew.
Both Taxol and Taxotere bind to cell microtubules, promoting their assembly into bundles and preventing cell mitosis. This eventually leads to the death of the cells. Although the mechanism of action for both drugs is the same, Taxotere has been found to be twice as potent as Taxol.
|
Molecular Playground Banner: "Paclitaxel (Taxol),a plant-derived natural product to treat cancer"
|
Molecular Playground Banner: "Docetaxel (Taxotere), an analaog of the plant derived anti-cancer agent paclitaxel"
|