Molecular Playground/E. coli ClpP
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== ClpP == | == ClpP == | ||
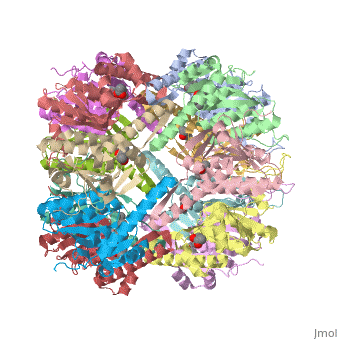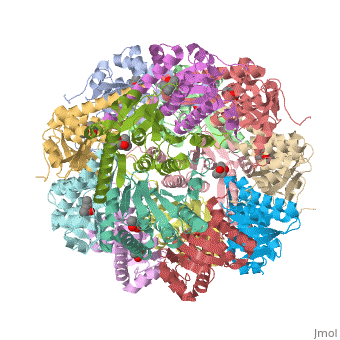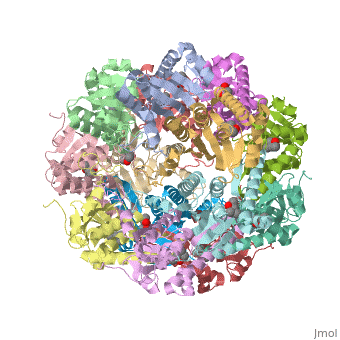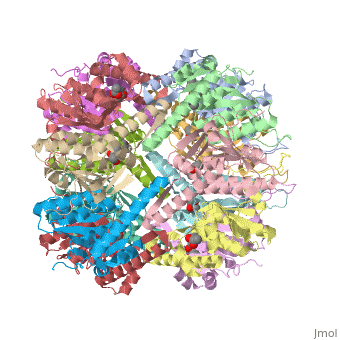
''E. coli'' Casein lytic proteinase P (ClpP) is a double ring tetradecameric homo oligomer compartmentalized peptidase [1]. ClpP requires the use of ATP dependent regulatory elements that independently bind to ClpP in order for substrates to have access the active core [2-4]. Here, proteins that are translocated by regulatory elements into the peptidase core are cleaved into smaller amino acid chains approximately on average 6-8aa in length [5]. | ''E. coli'' Casein lytic proteinase P (ClpP) is a double ring tetradecameric homo oligomer compartmentalized peptidase [1]. ClpP requires the use of ATP dependent regulatory elements that independently bind to ClpP in order for substrates to have access the active core [2-4]. Here, proteins that are translocated by regulatory elements into the peptidase core are cleaved into smaller amino acid chains approximately on average 6-8aa in length [5]. | ||
| - | == | + | == Tetradecameric Structure == |
| - | + | ClpP is a serine protease which consists of fourteen monomers situated into two heptameric rings seated on top of each other. In the center of the barrel-shaped chamber lies a core of fourteen peptide-cleaving active sites, restricted by the narrow entrance called the ''axial pore''. As peptides are processively threaded in the pore by the regulatory elements, the peptides are then degraded into smaller fragments as a result from ClpP cleavage. Smaller peptides are then released through small openings found around the equatorial interface of the two stacked rings. | |
== Relevance == | == Relevance == | ||
Revision as of 16:25, 3 December 2014
Here in the Chien lab, we study how degradation plays a large part in protein quality control. The maintenance and timely destruction of protein levels plays an important role during cell homeostasis and cell transitions/differentiation, yet much of what governs these processes has yet to be fully understood.
Introduction
| |||||||||||
References
Acknowledgements
Kamal Joshi, Joanne Lau, Jing Liu, Rob Vass