Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
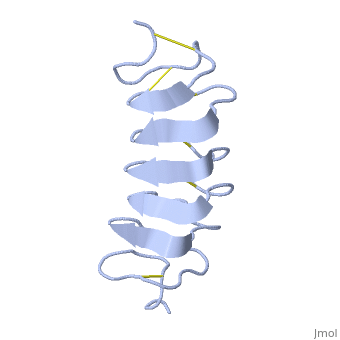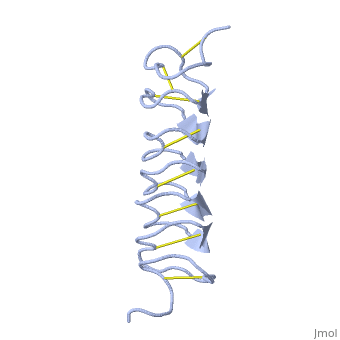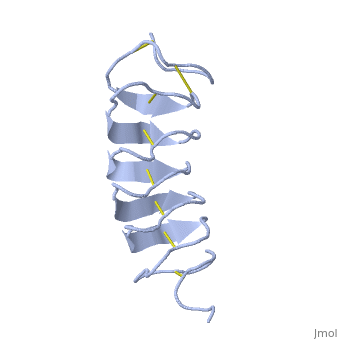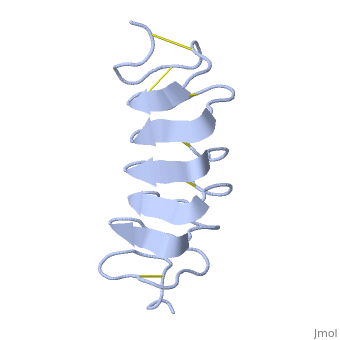
TmAFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle. | TmAFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle. | ||
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | ||
| - | TmAFP composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx). | + | TmAFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids-(TCTxSxxCxxAx). |
| - | TCT or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein. | + | TCT (theonine, cystein, threonine) or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein. |
The rest of the tandem repeat forms the loop which enables very organized structure of the protein. | The rest of the tandem repeat forms the loop which enables very organized structure of the protein. | ||
| - | Cysteine all over the tandem repeats, provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. | + | Cysteine all over the tandem repeats, are pared to provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. |
| - | six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> do not fit this pattern. | + | six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> in the N-terminal region do not fit this pattern. |
The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop hydrogen bond connections between backbone CO and NH groups. | The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop hydrogen bond connections between backbone CO and NH groups. | ||
Revision as of 15:01, 16 December 2014
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ Mitochondria as we don't know them. Tielens, Aloysius G.M et al. Trends in Biochemical Sciences , Volume 27 , Issue 11 , 564 - 572 doi:10.1016/S0968-0004(02)02193-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604