Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
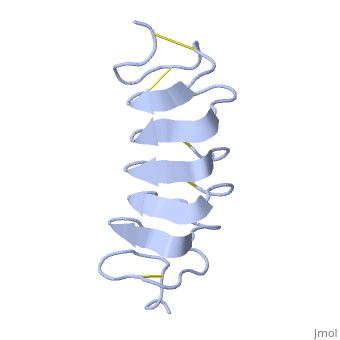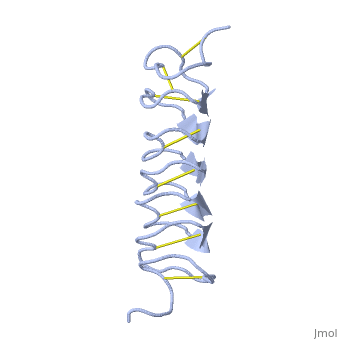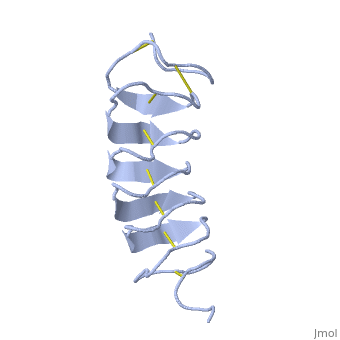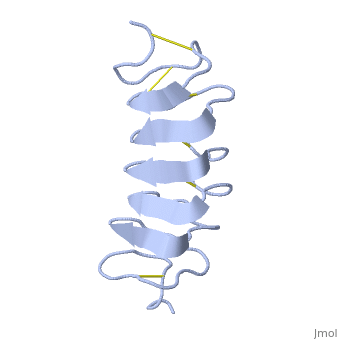
TmAFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. | TmAFP is the smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. | ||
| - | The few Hydrophobic residues <scene name='61/612804/Hydrophobic/ | + | The few Hydrophobic residues <scene name='61/612804/Hydrophobic/2'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.<ref>DOI 10.1038/35018604</ref> |
Revision as of 09:29, 21 December 2014
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ Mitochondria as we don't know them. Tielens, Aloysius G.M et al. Trends in Biochemical Sciences , Volume 27 , Issue 11 , 564 - 572 doi:10.1016/S0968-0004(02)02193-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604