Sandbox Reserved 820
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
<StructureSection load='2vaf' size='450' side='right'caption='Crystal Structure of Human Cardiac Calsequestrin, (PDB code [[2vaf]]) ' scene='56/568018/General_structure/4' > | <StructureSection load='2vaf' size='450' side='right'caption='Crystal Structure of Human Cardiac Calsequestrin, (PDB code [[2vaf]]) ' scene='56/568018/General_structure/4' > | ||
'''Calsequestrin-2''' (or '''CASQ2''') is the soluble Ca<sup>2+</sup> binding protein in the sarcoplasmic reticulum lumen of the cardiac muscle cells. CASQ2 could be either in a monomeric, homodimeric, or homooligomeric chain form depending on its bounds with Ca<sup>2+</sup>. Mutations of CASQ2 are involved in cardiac diseases such as Catecholaminergic Polymorphic Ventricular Tachycardia.<ref name="Cerrone">PMID:19879546</ref> | '''Calsequestrin-2''' (or '''CASQ2''') is the soluble Ca<sup>2+</sup> binding protein in the sarcoplasmic reticulum lumen of the cardiac muscle cells. CASQ2 could be either in a monomeric, homodimeric, or homooligomeric chain form depending on its bounds with Ca<sup>2+</sup>. Mutations of CASQ2 are involved in cardiac diseases such as Catecholaminergic Polymorphic Ventricular Tachycardia.<ref name="Cerrone">PMID:19879546</ref> | ||
| - | [[Image: | + | [[Image:.gif|350px|left|thumb|Calsequestrin in the calcium cycle of myocyte contraction]] |
{{clear}} | {{clear}} | ||
== Biological role == | == Biological role == | ||
Revision as of 08:25, 18 December 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Cerrone M, Napolitano C, Priori SG. Catecholaminergic polymorphic ventricular tachycardia: A paradigm to understand mechanisms of arrhythmias associated to impaired Ca(2+) regulation. Heart Rhythm. 2009 Nov;6(11):1652-9. doi: 10.1016/j.hrthm.2009.06.033. Epub 2009 , Jun 30. PMID:19879546 doi:http://dx.doi.org/10.1016/j.hrthm.2009.06.033
- ↑ NCBI Gene Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/gene/845
- ↑ Martin JL. Thioredoxin--a fold for all reasons. Structure. 1995 Mar 15;3(3):245-50. PMID:7788290
- ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?ascbin=8&maxaln=10&seltype=2&uid=239372&querygi=429544235&aln=1,227,0,109
- ↑ Polymerization of Calsequestrin: IMPLICATIONS FOR Ca2+ and REGULATION (Park et al., 2003) http://www.jbc.org/content/278/18/16176.full.pdf+html
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Crystal Structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum (Wang et al., 1998) http://www.nature.com/nsmb/journal/v5/n6/abs/nsb0698-476.html
- ↑ NCBI Structure Ressource: CASQ2 calsequestrin 2 http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi
- ↑ The Asp-rich region at the carboxyl-terminus of calsequestrin binds to Ca2+ and interacts with triadin (Shin et al., 2000) http://www.sciencedirect.com/science/article/pii/S0014579300022468
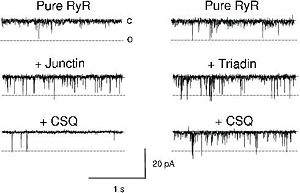
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004 May;85(1):33-69. PMID:15050380 doi:http://dx.doi.org/10.1016/j.pbiomolbio.2003.07.001
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005 May;88(5):3444-54. Epub 2005 Feb 24. PMID:15731387 doi:http://dx.doi.org/10.1529/biophysj.104.051441
Proteopedia page contributors and editors
Marc-Antoine JACQUES and Thomas VUILLEMIN