We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3sn6
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3sn6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3sn6 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3sn6 RCSB], [http://www.ebi.ac.uk/pdbsum/3sn6 PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3sn6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3sn6 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=3sn6 RCSB], [http://www.ebi.ac.uk/pdbsum/3sn6 PDBsum]</span></td></tr> | ||
</table> | </table> | ||
| + | == Function == | ||
| + | [[http://www.uniprot.org/uniprot/ADRB2_HUMAN ADRB2_HUMAN]] Beta-adrenergic receptors mediate the catecholamine-induced activation of adenylate cyclase through the action of G proteins. The beta-2-adrenergic receptor binds epinephrine with an approximately 30-fold greater affinity than it does norepinephrine. [[http://www.uniprot.org/uniprot/GNAS2_BOVIN GNAS2_BOVIN]] Guanine nucleotide-binding proteins (G proteins) are involved as modulators or transducers in various transmembrane signaling systems. The G(s) protein is involved in hormonal regulation of adenylate cyclase: it activates the cyclase in response to beta-adrenergic stimuli. [[http://www.uniprot.org/uniprot/GBG2_BOVIN GBG2_BOVIN]] Guanine nucleotide-binding proteins (G proteins) are involved as a modulator or transducer in various transmembrane signaling systems. The beta and gamma chains are required for the GTPase activity, for replacement of GDP by GTP, and for G protein-effector interaction. [[http://www.uniprot.org/uniprot/GBB1_RAT GBB1_RAT]] Guanine nucleotide-binding proteins (G proteins) are involved as a modulator or transducer in various transmembrane signaling systems. The beta and gamma chains are required for the GTPase activity, for replacement of GDP by GTP, and for G protein-effector interaction. | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
Revision as of 01:20, 25 December 2014
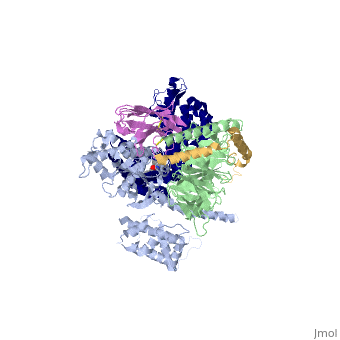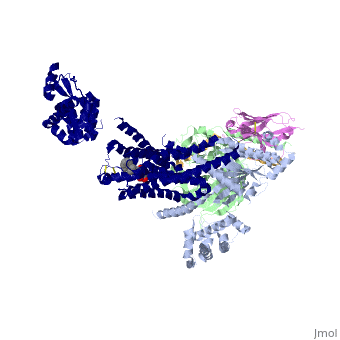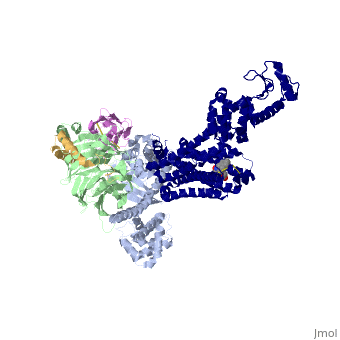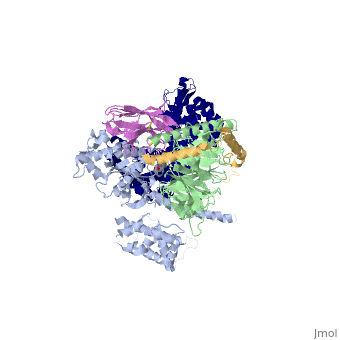
Crystal structure of the beta2 adrenergic receptor-Gs protein complex
| |||||||||||
Categories: Bos taurus | Enterobacteria phage t4 | Lama glama | Lysozyme | Rattus norvegicus | Caffrey, M | Calinski, D | Chae, P S | Chung, K Y | DeVree, B T | Gellman, S H | Kobilka, B K | Kobilka, T S | Kruse, A C | Lyons, J A | Mathiesen, J M | Pardon, E | Rasmussen, S G.F | Shah, S T.A | Skiniotis, G | Steyaert, J | Sunahara, R K | Thian, F S | Weis, W I | Zou, Y | G protein signaling | G protein-coupled receptor | Gpcr | Nanobody | Seven transmembrane receptor | Signal transduction | Signaling protein-hydrolase complex