Sandbox Reserved 959
From Proteopedia
| Line 34: | Line 34: | ||
Defensin-α-1 peptides are secreted inactive. They are cationic antimicrobial peptides that are synthesized in vivo as inactive precursors. Activation requires proteolytic excision of their anionic N-terminal inhibitory pro-peptide. The pro-peptide also specifically interacts with and inhibits the antimicrobial activity of the defensin-α-1 intermolecularly.<ref>www.ncbi.nlm.nih.gov/pmc/articles/PMC2754386/ )</ref> <br /> | Defensin-α-1 peptides are secreted inactive. They are cationic antimicrobial peptides that are synthesized in vivo as inactive precursors. Activation requires proteolytic excision of their anionic N-terminal inhibitory pro-peptide. The pro-peptide also specifically interacts with and inhibits the antimicrobial activity of the defensin-α-1 intermolecularly.<ref>www.ncbi.nlm.nih.gov/pmc/articles/PMC2754386/ )</ref> <br /> | ||
The active mature defensin-α-1 peptides consists of 29–35 amino acid residues with a molecular mass of 3–5 kDa. | The active mature defensin-α-1 peptides consists of 29–35 amino acid residues with a molecular mass of 3–5 kDa. | ||
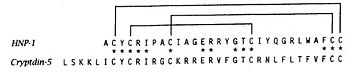
| - | The primary structure shows highly conserved residues, which are indispensable for the structural stability of the peptides. Among them are six invariant cysteine residues, necessary for the typical defensin-α-1 intramolecular disulphide-bond connectivity (Cys1–Cys6, Cys2–Cys4 and Cys3–Cys5). We can observe this bridge on the following figure. There are two charged amino acid residues, Arg5, and Glu13, forming a conserved salt bridge, and Gly17, which constitutes a signature structural motif which is essential for correct folding. | + | The primary structure shows highly conserved residues, which are indispensable for the structural stability of the peptides. Among them are six invariant cysteine residues, necessary for the typical defensin-α-1 intramolecular disulphide-bond connectivity |
| + | <scene name='60/604478/Residus-cysteine/1'>(Cys1–Cys6, Cys2–Cys4 and Cys3–Cys5)</scene>. We can observe this bridge on the following figure. There are two charged amino acid residues, Arg5, and Glu13, forming a conserved salt bridge, and Gly17, which constitutes a signature structural motif which is essential for correct folding. | ||
The tertiary structure is a triple-stranded β-sheet with a β-hairpin that contains cationic amino acid residues. | The tertiary structure is a triple-stranded β-sheet with a β-hairpin that contains cationic amino acid residues. | ||
<ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2049026/</ref>." | <ref>http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2049026/</ref>." | ||
Revision as of 12:44, 23 December 2014
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Introduction
Defensins (DEF) are a family of protein which are involved in host defenses in the epithelia of mucosal surfaces such as those of the intestin, respiratory tract, urinary tract, and vagina. There are antimicrobial and cytotoxic. All the protein of the family are distinguished by a cystein motif and are encoded on the chromozome 8.
[1]. There are many defensin but in this article we will focus on the defensin-α-1. The defensin-α-1 is a polypeptide which is found in the microbicidal granules of neutrophils. It's syntetisize in the paneth cell, which plays a role in the defense process. [2]It plays a role in phagocite-mediated host defense.
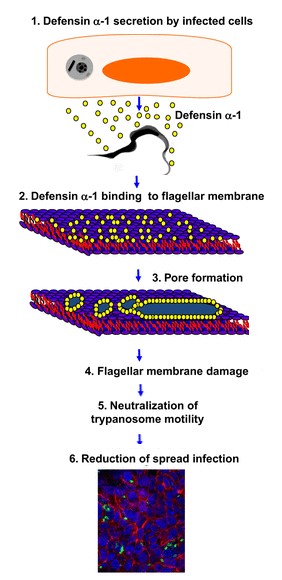
In this article, we will focus on the action of the defensin-α-1 against the "trypanosoma cruzy".
| |||||||||||