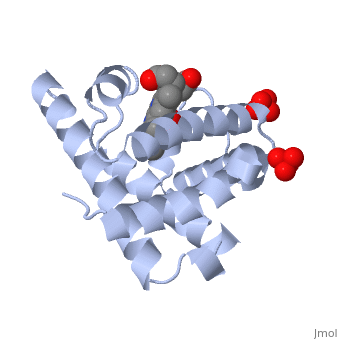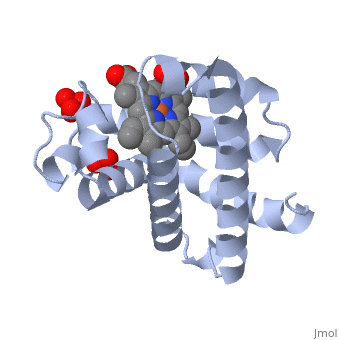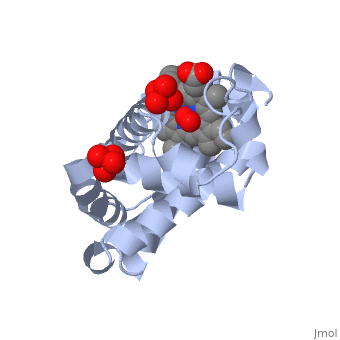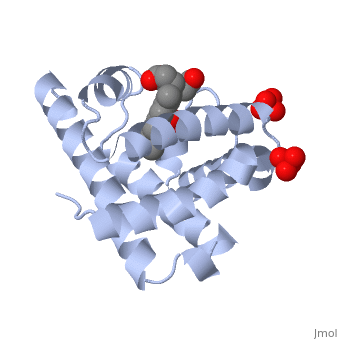Myoglobin
From Proteopedia
| Line 7: | Line 7: | ||
</td></tr></table> | </td></tr></table> | ||
| - | {{STRUCTURE_1a6m| PDB=1a6m | SIZE=400| SCENE='Myoglobin/Myoglobin/2 right'|CAPTION=Sperm whale myoglobin complex with O2 and sulfate, [[1a6m]] }} | ||
| + | <StructureSection load='1a6m' size='350' side='right' caption='Structure of Sperm whale myoglobin with O2 and sulfate (PDB entry [[1a6m]])' scene=''> | ||
[[Myoglobin]] is a globular protein whose function is to store molecular oxygen. | [[Myoglobin]] is a globular protein whose function is to store molecular oxygen. | ||
| - | + | ||
| - | + | ||
View1 shows a ribbon diagram, in gray, of the "Globin" (protein) portion of sperm whale deoxymyoglobin in which its eight helical segments, A through H, are displayed with two strands. Use the "zoom" slider to properly size the molecule in the viewer. Toggle the "ANIMATE" button to sequentially color these helices and their preceding nonhelical segments in rainbow order. You can see that the globin consists mostly of [[Helices in Proteins|alpha helices]]; it has no beta sheets and its nonhelical segments mostly serve as links that connect the helices. Look down the barrel of some of the longer helices. Are they all straight? | View1 shows a ribbon diagram, in gray, of the "Globin" (protein) portion of sperm whale deoxymyoglobin in which its eight helical segments, A through H, are displayed with two strands. Use the "zoom" slider to properly size the molecule in the viewer. Toggle the "ANIMATE" button to sequentially color these helices and their preceding nonhelical segments in rainbow order. You can see that the globin consists mostly of [[Helices in Proteins|alpha helices]]; it has no beta sheets and its nonhelical segments mostly serve as links that connect the helices. Look down the barrel of some of the longer helices. Are they all straight? | ||
The heme is shown, in pink, in wireframe form with its N, O, and Fe atoms displayed as blue, red, and orange balls. Note how the heme is almost completely enclosed by the globin. Which few chemical groups of the Heme are exposed to the solvent? (Clicking on atoms displays their identity in the lower left hand corner.) Can you rationalize this exposure? | The heme is shown, in pink, in wireframe form with its N, O, and Fe atoms displayed as blue, red, and orange balls. Note how the heme is almost completely enclosed by the globin. Which few chemical groups of the Heme are exposed to the solvent? (Clicking on atoms displays their identity in the lower left hand corner.) Can you rationalize this exposure? | ||
| Line 22: | Line 21: | ||
Myoglobin page in Spanish [[Myoglobin-Physeter-catodon-structure]]. | Myoglobin page in Spanish [[Myoglobin-Physeter-catodon-structure]]. | ||
| + | </StructureSection> | ||
</font> | </font> | ||
| - | + | Former exercise in large part by John H. Connor (present address: Department of Microbiology, Boston University School of Medicine, 850 Harrison Ave, Boston, MA, 02118, USA). Revised by Ann Taylor | |
== 3D Structures of Myoglobin == | == 3D Structures of Myoglobin == | ||
Revision as of 03:06, 29 March 2015
|
Caution: The text in this article and has not been updated to reflect what is actually available on this page. There is no zoom slider and no animate button. These were formerly present when an earlier version of Proteopedia supported Kinemages. A volunteer is needed to clean up and improve this article on a pedagogically and historically important protein. Green links are needed! Text referring to the now absent Kinemage is in gray. Eric Martz 01:09, 13 September 2014 (IDT) |
| |||||||||||
</font>
Former exercise in large part by John H. Connor (present address: Department of Microbiology, Boston University School of Medicine, 850 Harrison Ave, Boston, MA, 02118, USA). Revised by Ann Taylor
3D Structures of Myoglobin
Updated on 29-March-2015 Myoglobin (Mb) is an oxygen binding protein found in muscle tissue. It contains a heme group. Metmyoglobin (MMb) is the oxidized form of myoglobin.
((3qm5, 3qm6 – btMb – blackfin tuna
External Resources
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Ann Taylor, Alexander Berchansky, Joel L. Sussman, Eric Martz, Jaime Prilusky, Karsten Theis, Karl Oberholser, Eran Hodis, Judy Voet, David Canner