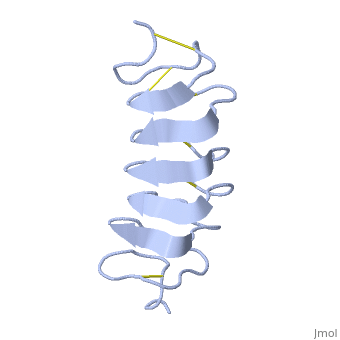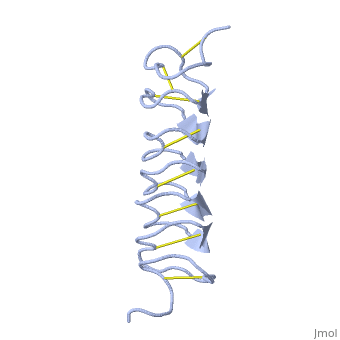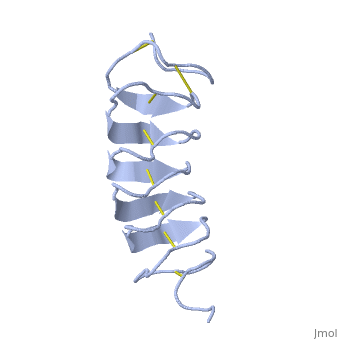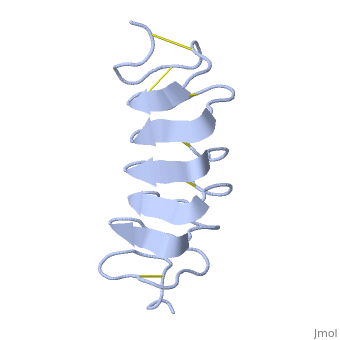
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
| - | + | ||
== Function == | == Function == | ||
| - | The two dimensional arrays of Threonine side chain makes a remarkably good match to the repeated spacing between oxygen atoms in the ice lattice on the primary prism plane, and a reasonable match to the basal plane-This is why the activity of ''Tm''AFP ( Thermal hysteresis) is much higher than the acticity of AFP from Fish (5-10 celcius degrees and 1.5 celcius degrees respectively) | + | The two dimensional arrays of Threonine side chain makes a remarkably good match to the repeated spacing between oxygen atoms in the ice lattice on the primary prism plane, and a reasonable match to the basal plane-This is why the activity of ''Tm''AFP ( Thermal hysteresis) is much higher than the acticity of AFP from Fish (5-10 celcius degrees and 1.5 celcius degrees respectively)<ref>DOI 10.1016/S0968-0004</ref> |
In solution the protein is monomeric but it crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>. | In solution the protein is monomeric but it crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>. | ||
Revision as of 09:29, 6 January 2015
| |||||||||||
References
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ Mitochondria as we don't know them. Tielens, Aloysius G.M et al. Trends in Biochemical Sciences , Volume 27 , Issue 11 , 564 - 572 doi:10.1016/S0968-0004(02)02193-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604