We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 972
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
==Context== | ==Context== | ||
| - | <scene name='60/604491/Ide/1'>Insulin Degrading Enzyme</scene>(or IDE) | + | <scene name='60/604491/Ide/1'>Insulin Degrading Enzyme</scene>(or IDE) plays an important role in the cellular degradation of many molecules. Its activity was first related only to insulin, but now, we know that IDE interacts with insulin, glugacon and bradykinin. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
The IDE-bradykinin complex has a complexity in the substrate binding and recognition mechanism of IDE. | The IDE-bradykinin complex has a complexity in the substrate binding and recognition mechanism of IDE. | ||
| Line 20: | Line 16: | ||
===Exosite: an essential element for the catalysis=== | ===Exosite: an essential element for the catalysis=== | ||
This is an essential component for the substrate hydrolysis. It plays a role in the docking of substrates on IDE. Actually, when substrates arrive, they first bind to the exosite and then, they are translocated to the catalytic site. They have to change their conformation in order to access to the catalytic site. | This is an essential component for the substrate hydrolysis. It plays a role in the docking of substrates on IDE. Actually, when substrates arrive, they first bind to the exosite and then, they are translocated to the catalytic site. They have to change their conformation in order to access to the catalytic site. | ||
| - | |||
===Catalytic mechanism=== | ===Catalytic mechanism=== | ||
| Line 26: | Line 21: | ||
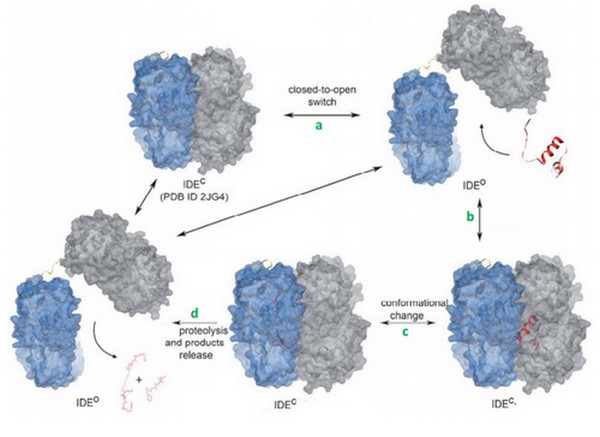
The catalytic mechanism occurs in 4 steps: | The catalytic mechanism occurs in 4 steps: | ||
| - | 1. IDE changes its state from close to open. This permitts the binding of the substrate in the catalytic chamber. | + | 1. IDE changes its state from close to open. This permitts the binding of the substrate in the catalytic chamber. |
| - | 2. The IDE-substrate complex changes into close conformation. | + | 2. The IDE-substrate complex changes into close conformation. |
| - | 3. The substrate can now bind to the exosite with his N-term tail and then change his conformation in order to be acceptable for the catalytic site. IDE can now cleave the substrate. | + | 3. The substrate can now bind to the exosite with his N-term tail and then change his conformation in order to be acceptable for the catalytic site. IDE can now cleave the substrate. |
| - | 4. IDE changes into open conformation and release products. | + | 4. IDE changes into open conformation and release products. |
[[Image:Proteo.jpg]] | [[Image:Proteo.jpg]] | ||
| - | |||
==Bradykinin== | ==Bradykinin== | ||
| Line 41: | Line 35: | ||
Today, we know that kinins can be degraded by IDE. | Today, we know that kinins can be degraded by IDE. | ||
| - | |||
| - | |||
| - | We supposed that IDE may bind two molecules of bradykinin at the same time | ||
==Recognition and interactions between bradykinin and IDE== | ==Recognition and interactions between bradykinin and IDE== | ||
Revision as of 20:31, 9 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644