Sandbox Reserved 972
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Insuline degrading enzyme== | ==Insuline degrading enzyme== | ||
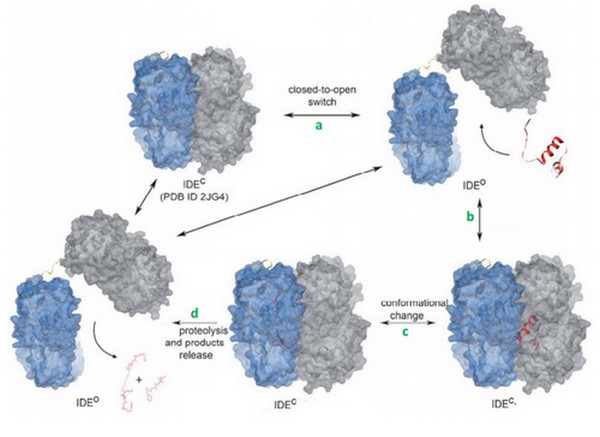
| - | <scene name='60/604491/Ide/1'>IDE</scene> (EC 3.4.24.56) is a human enzyme of the metallopeptidase family. It is composed by more than 1000 residues and has a huge catalytic cavity. It is made of 2 parts linked by a loop, and it switches between an open and a close state. The size of its catalytic chamber allows the binding of peptides (70 amino acids long). IDE hydrolyzes a lot of substrates which have many differents biological activities. Its substrate can be insuline, glucagon, amyline or bradykinin. This enzyme hydrolyses its substrates by cleaving them at different points. | + | <scene name='60/604491/Ide/1'>IDE</scene> |
| + | [http://www.proteopedia.org/wiki/index.php/Insulin-Degrading_Enzyme] | ||
| + | (EC 3.4.24.56) is a human enzyme of the metallopeptidase family. It is composed by more than 1000 residues and has a huge catalytic cavity. It is made of 2 parts linked by a loop, and it switches between an open and a close state. The size of its catalytic chamber allows the binding of peptides (70 amino acids long). IDE hydrolyzes a lot of substrates which have many differents biological activities. Its substrate can be insuline, glucagon, amyline or bradykinin. This enzyme hydrolyses its substrates by cleaving them at different points. | ||
===Exosite: an essential element for the catalysis=== | ===Exosite: an essential element for the catalysis=== | ||
| Line 42: | Line 44: | ||
The degradation of kinins and specially bradykinin is not well understand yet. The cleavage site of bradykinin by IDE, the detailed kinetic analysis and the structural basis for recognition have to be more studied. | The degradation of kinins and specially bradykinin is not well understand yet. The cleavage site of bradykinin by IDE, the detailed kinetic analysis and the structural basis for recognition have to be more studied. | ||
| - | |||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 22:16, 9 January 2015
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Im H, Manolopoulou M, Malito E, Shen Y, Zhao J, Neant-Fery M, Sun CY, Meredith SC, Sisodia SS, Leissring MA, Tang WJ. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J Biol Chem. 2007 Aug 31;282(35):25453-63. Epub 2007 Jul 5. PMID:17613531 doi:10.1074/jbc.M701590200
- ↑ Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006 Oct 19;443(7113):870-4. Epub 2006 Oct 11. PMID:17051221 doi:10.1038/nature05143
- ↑ Song ES, Juliano MA, Juliano L, Hersh LB. Substrate activation of insulin-degrading enzyme (insulysin). A potential target for drug development. J Biol Chem. 2003 Dec 12;278(50):49789-94. Epub 2003 Oct 2. PMID:14527953 doi:http://dx.doi.org/10.1074/jbc.M308983200
- ↑ Im H, Manolopoulou M, Malito E, Shen Y, Zhao J, Neant-Fery M, Sun CY, Meredith SC, Sisodia SS, Leissring MA, Tang WJ. Structure of substrate-free human insulin-degrading enzyme (IDE) and biophysical analysis of ATP-induced conformational switch of IDE. J Biol Chem. 2007 Aug 31;282(35):25453-63. Epub 2007 Jul 5. PMID:17613531 doi:10.1074/jbc.M701590200
- ↑ Malito E, Ralat LA, Manolopoulou M, Tsay JL, Wadlington NL, Tang WJ. Molecular Bases for the Recognition of Short Peptide Substrates and Cysteine-Directed Modifications of Human Insulin-Degrading Enzyme. Biochemistry. 2008 Nov 6. PMID:18986166 doi:10.1021/bi801192h