We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 976
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Tissue Plasminogen Activator (t-PA)== | ==Tissue Plasminogen Activator (t-PA)== | ||
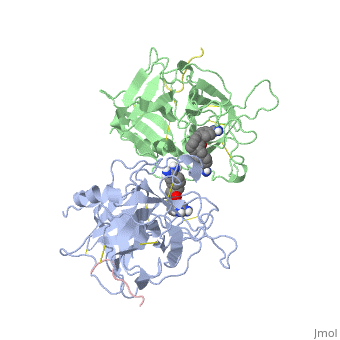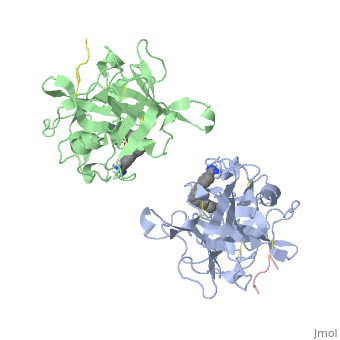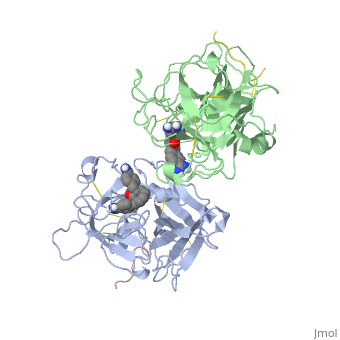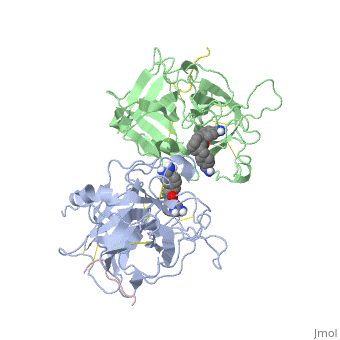
<StructureSection load='1a5h' size='340' side='right' caption='Stereo ribbon plot of the catalytic domain of t-PA in complex with the bis-benzamidine inhibitor' scene=''> | <StructureSection load='1a5h' size='340' side='right' caption='Stereo ribbon plot of the catalytic domain of t-PA in complex with the bis-benzamidine inhibitor' scene=''> | ||
| - | Tissue-type Plasminogen Activator – usually called t-PA – is a trypsin-like serine protease that catalyses the conversion of zymogen plasminogen into plasmin. Once present in the blood, plasmin – which is an active enzyme – degrades fibrin clots, the product of a coagulation. tPA is clinically used as a treatment for acute ischemic strokes, myocardial infarction and pulmonary embolism. | + | Tissue-type Plasminogen Activator – usually called t-PA – is a trypsin-like serine protease that catalyses the conversion of [http://en.wikipedia.org/wiki/Zymogen zymogen] plasminogen into [[plasmin]]. Once present in the blood, plasmin – which is an active enzyme – degrades [[fibrin]] clots, the product of a coagulation. tPA is clinically used as a treatment for acute [http://en.wikipedia.org/wiki/Stroke#Ischemic ischemic strokes], myocardial infarction and pulmonary embolism. |
== Interactions with other proteins == | == Interactions with other proteins == | ||
t-PA proteins interacts with LRPs (lipoprotein receptor-related protein) which are produced in endothelial cells. This interaction leads to NFkB (Nuclear Factor-Kappa B) activation and MMPs (Matrix metalloproteinases) overexpression, by the activation of latent PDGF-CC (Platelet-derived growth factor receptors).<ref name=vivien>Vivien D, Gauberti M, Montagne A, Defer G and Touzé E (2011) Impact of tissue plaminogen activator on the neurovascular unit : from clinical data to experimental evidence. Journal of Cerebral Blood Flow & Metabolism, 31 : 2119-2134.</ref> | t-PA proteins interacts with LRPs (lipoprotein receptor-related protein) which are produced in endothelial cells. This interaction leads to NFkB (Nuclear Factor-Kappa B) activation and MMPs (Matrix metalloproteinases) overexpression, by the activation of latent PDGF-CC (Platelet-derived growth factor receptors).<ref name=vivien>Vivien D, Gauberti M, Montagne A, Defer G and Touzé E (2011) Impact of tissue plaminogen activator on the neurovascular unit : from clinical data to experimental evidence. Journal of Cerebral Blood Flow & Metabolism, 31 : 2119-2134.</ref> | ||
| Line 7: | Line 7: | ||
t-Pa also interacts with the N-methyl-D-aspartate (NDMA) receptor, and annexin-II which are both in glial cells and neurons. This interactions leads to deleterious effects because it results in parenchymal cell death. <ref name=vivien/> | t-Pa also interacts with the N-methyl-D-aspartate (NDMA) receptor, and annexin-II which are both in glial cells and neurons. This interactions leads to deleterious effects because it results in parenchymal cell death. <ref name=vivien/> | ||
| - | t-Pa induces the overactivaton of MMP-3 (the effector arm of the change of cerebrovascular permeability | + | t-Pa induces the overactivaton of MMP-3 (the effector arm of the change of cerebrovascular permeability <ref name=maithili>Maithili Sashindranath,1 Eunice Sales,1 Maria Daglas,1 Roxann Freeman, (2012) The tissue-type plasminogen activator–plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans. Brain 2012: 135; 3251–3264</ref>) and MMP-9, which are two marker proteins of the Brain Blood Barrier (the current research on t-PA are focused on finding a way to stop this overactivation).<ref name=vivien/> |
The deleterious effects of t-PA is all about the interaction with LRP, so a combine therapy of tPA and LRP antagonist could be useful for clinical treatments <ref name=vivien/>, but currently no evidences of this type of treatment has been published. | The deleterious effects of t-PA is all about the interaction with LRP, so a combine therapy of tPA and LRP antagonist could be useful for clinical treatments <ref name=vivien/>, but currently no evidences of this type of treatment has been published. | ||
== Medical uses and Clinical evidences == | == Medical uses and Clinical evidences == | ||
| - | Numerous clinical test were performed on t-PA, this molecule is currently the unique treatment for acute ischemic stroke. But as noticed in the previous section, t-PA is associated with increased risk of cerebral haemorrhage and brain injury. | + | Numerous clinical test were performed on t-PA, this molecule is currently the unique treatment for acute ischemic stroke. But as noticed in the previous section, t-PA is associated with increased risk of cerebral haemorrhage and brain injury. <ref name=yasuhiro>Role of Tissue-Type Plasminogen Activator in Ischemic Stroke, Yasuhiro Suzuki 1, (2010) J Pharmacol Sci 113, 203 – 207 (2010)</ref>. |
| - | In case of mouse experimentation, the FCII (focal cerebral ischemic injury) size was smaller in the WT (Wild Type) mice than in the t-PA −/− | + | In case of mouse experimentation, the FCII (focal cerebral ischemic injury) size was smaller in the WT (Wild Type) mice than in the t-PA −/−. <ref name=yasuhiro/> |
| - | Otherwise, the treatment of PVT (Portal vein thrombosis) in pregnant woman have success rate (but induces foetal mortality of 20%). | + | Otherwise, the treatment of PVT (Portal vein thrombosis) in pregnant woman have success rate (but induces foetal mortality of 20%).<ref name=mehmet>Thrombolytic Therapy for the Treatment of Prosthetic Heart Valve Thrombosis in Pregnancy With Low-Dose, Slow Infusion of Tissue-Type Plasminogen Activator (2013). Mehmet Özkan, Beytullah Çakal, Süleyman Karakoyun, Ozan Mustafa Gürsoy, doi: 10.1161/CIRCULATIONAHA.113.001145 Circulation. 2013;128:532-540; originally published online June 28, 2013</ref> |
With advances in Bio-Engineering, several generation of tPA derivatives have progressively reached clinical trials but none has demonstrated benefits so far.<ref name=vivien/> | With advances in Bio-Engineering, several generation of tPA derivatives have progressively reached clinical trials but none has demonstrated benefits so far.<ref name=vivien/> | ||
== Structure of the catalytic domain of human tPA complexed with Bis-Benzamidine == | == Structure of the catalytic domain of human tPA complexed with Bis-Benzamidine == | ||
| - | The tissue-type Plasminogen Activator is a 69-kDa multidomain glycoprotein, which consists in a single polypeptide chain of 527-530 amino acids <ref name=vivien/>. There is three types of domain: F1 (homologous to fibronectin type I), G (epidermal growth-factor like) and kringle. | + | The tissue-type Plasminogen Activator is a 69-kDa multidomain glycoprotein, which consists in a single polypeptide chain of 527-530 amino acids <ref name=vivien/>. There is three types of domain: F1 (homologous to fibronectin type I), G (epidermal growth-factor like) and kringle.<ref name=smith>Smith BO, Downing AK, Driscoll PC, Dudgeon TJ, Campbell ID (1995) The solution structure ans backbone dynamics of the fibronectin type I and epidermal growth factor-like pair of modules of tissue-type plasminogen activator. Structure, 3 : 823-833</ref> |
| - | The structures described below correspond to the structure of the catalytic domain of humain tPA in complex with a Bis-Benzamidine. The catalytic domain of a protein is the part of the enzyme that reacts with the substrate to induce the enzymatic reaction and benzamidine is an organic compound often used as an inhibitor.. Thus, the complexation of t-PA with bis-benzamidine revealed a strong structural similarities to other trypsin-like serine proteases, as alpha-chymotrypsine. tPA is known for its high specificity : tPA recognizes complexes or multiple elements on the surface of plasminogen, even distant form its cleavage site. In vivo, the tPA cleavage site is a single bond of plasminogen formed by the residues Arg560-Val561 | + | The structures described below correspond to the structure of the catalytic domain of humain tPA in complex with a Bis-Benzamidine. The catalytic domain of a protein is the part of the enzyme that reacts with the substrate to induce the enzymatic reaction and benzamidine is an organic compound often used as an inhibitor.. Thus, the complexation of t-PA with bis-benzamidine revealed a strong structural similarities to other trypsin-like serine proteases, as alpha-chymotrypsine. tPA is known for its high specificity : tPA recognizes complexes or multiple elements on the surface of plasminogen, even distant form its cleavage site. In vivo, the tPA cleavage site is a single bond of plasminogen formed by the residues Arg560-Val561 <ref name=renatus >Renatus M, Bode W, Huber R, Stürzebecher J, Prasa D, Fisher S, Kohnert U, Stubbs MT (1997) Structural Mapping of the Active Site Specificity Determinants of Human Tissue-type Plasminogen Activator. The Journal of Biological Chemestry, 272 : 21712-21719</ref>. |
Few t-PA-specific inhibitors are known. Synthetic inhibitors for t-PA-like proteins has been based on Arigin and Lysin residues derivatives and the structurally related benzamidines. | Few t-PA-specific inhibitors are known. Synthetic inhibitors for t-PA-like proteins has been based on Arigin and Lysin residues derivatives and the structurally related benzamidines. | ||
| - | Many specific features of the protein involve interactions with surface elements of the catalytic domain. By the way, the two-chain tPA shows almost no structural differences when complexed with bis-benzamidine compound. The binding of the bis-benzamidine to tPA is determined by two interaction sites. The first group binds in the specificity pocket while the second group fits in an hydrophobic groove, resulting in an extended binding of the inhibitor | + | Many specific features of the protein involve interactions with surface elements of the catalytic domain. By the way, the two-chain tPA shows almost no structural differences when complexed with bis-benzamidine compound. The binding of the bis-benzamidine to tPA is determined by two interaction sites. The first group binds in the specificity pocket while the second group fits in an hydrophobic groove, resulting in an extended binding of the inhibitor <ref name=renatus/>. |
The tPA complexed with bis-benzamidine structure shows that the side chain of the Arg174 residue can adopt multiple conformations. | The tPA complexed with bis-benzamidine structure shows that the side chain of the Arg174 residue can adopt multiple conformations. | ||
| - | Asp 174 at the bottom of S1 specificity-pocket results in a negatively charged environment. The S4 pocket, delimited by the side chain of Tyr99 at its eastern side, Trp215 at the bottom and the aliphatic part of Arg174 at its western border, provide the entrance frame hydrophobic. The negative cavity is form at the north of the S4 pocket. The major determinants for interactions with substrates and inhibitors would appear to be binding in the hydrophobic S4 substrate, together with weak electrostatic interactions at the electronegativity cavity. | + | Asp 174 at the bottom of S1 specificity-pocket results in a negatively charged environment. The S4 pocket, delimited by the side chain of Tyr99 at its eastern side, Trp215 at the bottom and the aliphatic part of Arg174 at its western border, provide the entrance frame hydrophobic. The negative cavity is form at the north of the S4 pocket. The major determinants for interactions with substrates and inhibitors would appear to be binding in the hydrophobic S4 substrate, together with weak electrostatic interactions at the electronegativity cavity.<ref name=renatus/> |
== See also == | == See also == | ||
| - | + | [[Plasmin]] | |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:09, 11 January 2015
Tissue Plasminogen Activator (t-PA)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Vivien D, Gauberti M, Montagne A, Defer G and Touzé E (2011) Impact of tissue plaminogen activator on the neurovascular unit : from clinical data to experimental evidence. Journal of Cerebral Blood Flow & Metabolism, 31 : 2119-2134.
- ↑ Maithili Sashindranath,1 Eunice Sales,1 Maria Daglas,1 Roxann Freeman, (2012) The tissue-type plasminogen activator–plasminogen activator inhibitor 1 complex promotes neurovascular injury in brain trauma: evidence from mice and humans. Brain 2012: 135; 3251–3264
- ↑ 3.0 3.1 Role of Tissue-Type Plasminogen Activator in Ischemic Stroke, Yasuhiro Suzuki 1, (2010) J Pharmacol Sci 113, 203 – 207 (2010)
- ↑ Thrombolytic Therapy for the Treatment of Prosthetic Heart Valve Thrombosis in Pregnancy With Low-Dose, Slow Infusion of Tissue-Type Plasminogen Activator (2013). Mehmet Özkan, Beytullah Çakal, Süleyman Karakoyun, Ozan Mustafa Gürsoy, doi: 10.1161/CIRCULATIONAHA.113.001145 Circulation. 2013;128:532-540; originally published online June 28, 2013
- ↑ Smith BO, Downing AK, Driscoll PC, Dudgeon TJ, Campbell ID (1995) The solution structure ans backbone dynamics of the fibronectin type I and epidermal growth factor-like pair of modules of tissue-type plasminogen activator. Structure, 3 : 823-833
- ↑ 6.0 6.1 6.2 Renatus M, Bode W, Huber R, Stürzebecher J, Prasa D, Fisher S, Kohnert U, Stubbs MT (1997) Structural Mapping of the Active Site Specificity Determinants of Human Tissue-type Plasminogen Activator. The Journal of Biological Chemestry, 272 : 21712-21719