Inositol 1,4,5-Trisphosphate Receptor
From Proteopedia
| Line 4: | Line 4: | ||
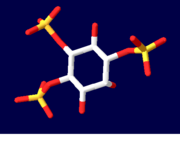
[[Inositol 1,4,5-Trisphosphate Receptor]] binding protein is a ubiquitous protein involved in the Ca<sup>2+</sup> signalling processes in a variety of organisms <ref name="mainpaper">PMID:12442173</ref> | [[Inositol 1,4,5-Trisphosphate Receptor]] binding protein is a ubiquitous protein involved in the Ca<sup>2+</sup> signalling processes in a variety of organisms <ref name="mainpaper">PMID:12442173</ref> | ||
__NOTOC__ | __NOTOC__ | ||
| - | == | + | ==Structure== |
| - | <StructureSection load='3rec' size='350' side='right' caption='Escherichia coli reca protein-bound DNA (PDB entry [[3rec]])' scene=''> | + | <StructureSection load='3rec' size='350' side='right' caption='Escherichia coli reca protein-bound DNA (PDB entry [[3rec]])' scene=''> |
| - | + | ||
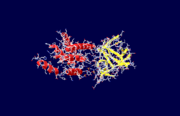
| - | + | The specific type of inositol 1,4,5-trisphosphate receptor (InsP<sub>3</sub>R) protein discussed here is the mouse type 1 InsP<sub>3</sub>R, also called InsP<sub>3</sub>R1. This polypeptide contains three major regions: the amino terminal inositol 1,4,5-trisphosphate (InsP<sub>3</sub>) binding region, the central modulatory region, and the carboxy-terminus channel region.<ref name="mainpaper"/> The protein forms an L-shaped structure composed of two asymmetric domains perpendicular to each other.<ref name="mainpaper"/> The N-terminal domain is made up of 12 β-strands and 2 single-turn helices, which come together to form a barrel.<ref name="mainpaper"/> The C-terminal end is quite different, consisting of a bundle made of eight α-helices.<ref name="mainpaper"/> The interface of the two domains is lined with basic residues and forms the receptor site for InsP<sub>3</sub>.<ref name="mainpaper"/> The InsP<sub>3</sub>R protein does not belong to a superfamily of proteins. The receptor is thought to span the membrane 6 times, leaving the C-terminus in the cytoplasm.<ref name="functionref"/> | |
=== Domain Structure === | === Domain Structure === | ||
Revision as of 04:46, 13 January 2015
Template:STRUCTURE 1n4k
Inositol 1,4,5-Trisphosphate Receptor binding protein is a ubiquitous protein involved in the Ca2+ signalling processes in a variety of organisms [1]
Structure
| |||||||||||
Function
Role in Ca2+ regulation
The presence of inositol 1,4,5-trisphosphate functions to increase the cytosolic concentration of Ca2+.[2] The InsP3 is formed at the plasma membrane, diffuses into the cytosol, and binds to the InsP3 receptor which is found in the membrane of intracellular Ca2+ stores.[2] The release of Ca2+ can propagate to other cells and can help to coordinate the functionality of organ systems.[2] Areas of the body rich in the InsP3 receptor are the cerebellum and, more specifically, the endoplasmic reticulum, and even the plasma membrane and nuclei of some tissues.[2] Recent results also suggest that InsP3 receptors work in intrinsic Ca2+ channel activity.[2]
Regulation of receptor activity
Sequences within the receptor protein have been found to interact with accessory proteins. Additionally, there are sites for ATP binding and for phosphorylation.[2] All of these interactions would play a role in the regulation of the InsP3 receptor protein.
A very important property of the receptor is that it is regulated by Ca2+ concentrations. Lower concentrations make the receptor more sensitive to InsP3 while high concentrations can inhibit the receptor activity.[2] Also, the receptor itself can bind Ca3 itself at more than one site. A Ca2+ binding site within the ligand binding domain may even suggest that these Ca2+ binding sites are involved in the effects Ca2+ has on InsP3 binding to its ligand.
The method of regulation by ATP on the receptor is very similar to that of Ca2+. Increased ATP concentrations increase receptor activity whereas higher concentrations decrease receptor activity.[2] The stimulatory activity of ATP likely occurs through consensus adenine nucleotide-binding motifs.[2] The inhibitory effect of ATP is thought to arise through its charged nature, acting as a competitive antagonist at the InsP3-binding site.[2]
The InsP3R protein can autophosphorylate itself and is a substrate for multiple protein kinases.[2] These kinases include cyclic AMP-dependent protein kinase (PKA), cyclic GMP-dependent protein kinase (PKG) and others.[2] The protein kinases are thought to interact with the InsP3 receptor by controlling the sensitivity to Ca2+ in different tissues as well as affecting the sensitivity of InsP3 itself to Ca2+.[2]
3D structures of inositol 1,4,5-trisphosphate receptor
Updated June 2012
3jrr – mInsP3R III ligand-binding suppressor domain – mouse
1xzz - mInsP3R I ligand-binding suppressor domain
1n4k - mInsP3R I receptor-binding core + ligand
3t8s, 3uj4 - rInsP3R I ligand-binding domain - rat
3uj0 - rInsP3R I ligand-binding domain + ligand
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002 Dec 12;420(6916):696-700. Epub 2002 Nov 17. PMID:12442173 doi:10.1038/nature01268
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999 Mar;25(3):247-64. PMID:10378086 doi:10.1054/ceca.1999.0021
Proteopedia Page Contributors and Editors (what is this?)
Shannon King, Alexander Berchansky, Michal Harel, Ann Taylor, David Canner, Andrea Gorrell, Jaclyn Gordon