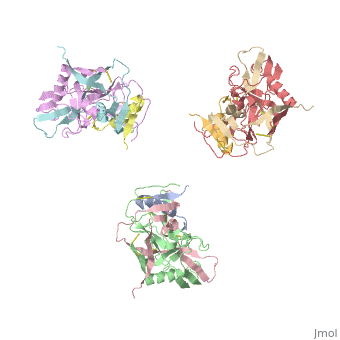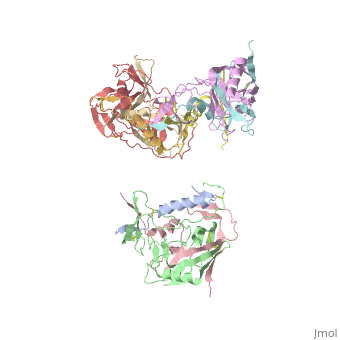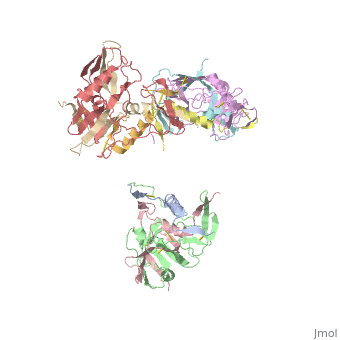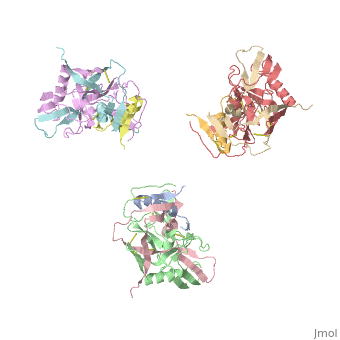We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hiv env proteins
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load='3dnn' size=' | + | <StructureSection load='3dnn' size='350' side='right' caption='Molecular structure for the HIV-1 gp120 trimer in the unliganded state (PDB entry [[3dnn]])' scene=''> |
== Introduction == | == Introduction == | ||
Human Immunodeficiency Virus (HIV) is a retrovirus/lentivirus that expresses a spike protein on its viral envelope '''(Env)'''. "The envelope protein is synthesized as a precursor, gp160, which trimerizes and undergoes cleavage into two noncovalently associated fragments, the receptor-binding fragment gp120 and the fusion fragment gp41" <ref name=Guan>PMID: 21076402 </ref>. Thus, Env is a heterodimer consisting of '''gp120''' and '''gp41''' and forms trimers on the surface of the viral membrane. As "the sole antigen on the virion surface," an understanding of Env (gp 120 & 41) and the conformational changes that occur in gp120 and gp41 during binding and fusion, respectively, is necessary in developing new fusion inhibitors and possible vaccines <ref name=Guan>PMID: 21076402 </ref> <ref name=Nick>PMID: 18668044 </ref>. The protein structure shown on the right is the gp120 portion of Env in its unbound trimer state <ref name=Nick>PMID: 18668044 </ref>. | Human Immunodeficiency Virus (HIV) is a retrovirus/lentivirus that expresses a spike protein on its viral envelope '''(Env)'''. "The envelope protein is synthesized as a precursor, gp160, which trimerizes and undergoes cleavage into two noncovalently associated fragments, the receptor-binding fragment gp120 and the fusion fragment gp41" <ref name=Guan>PMID: 21076402 </ref>. Thus, Env is a heterodimer consisting of '''gp120''' and '''gp41''' and forms trimers on the surface of the viral membrane. As "the sole antigen on the virion surface," an understanding of Env (gp 120 & 41) and the conformational changes that occur in gp120 and gp41 during binding and fusion, respectively, is necessary in developing new fusion inhibitors and possible vaccines <ref name=Guan>PMID: 21076402 </ref> <ref name=Nick>PMID: 18668044 </ref>. The protein structure shown on the right is the gp120 portion of Env in its unbound trimer state <ref name=Nick>PMID: 18668044 </ref>. | ||
Revision as of 10:37, 8 February 2016
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Frey G, Chen J, Rits-Volloch S, Freeman MM, Zolla-Pazner S, Chen B. Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies. Nat Struct Mol Biol. 2010 Dec;17(12):1486-91. Epub 2010 Nov 14. PMID:21076402 doi:10.1038/nsmb.1950
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008 Sep 4;455(7209):109-13. Epub 2008 Jul 30. PMID:18668044 doi:10.1038/nature07159
- ↑ 3.0 3.1 3.2 Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998 Jun 18;393(6686):648-59. PMID:9641677 doi:10.1038/31405
- ↑ 4.0 4.1 4.2 4.3 Chen L, Do Kwon Y, Zhou T, Wu X, O'Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, Yang ZY, Zhang MY, Arthos J, Burton DR, Dimitrov DS, Nabel GJ, Posner MR, Sodroski J, Wyatt R, Mascola JR, Kwong PD. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009 Nov 20;326(5956):1123-7. PMID:19965434 doi:326/5956/1123
- ↑ Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, Lambson BE, Ranchobe N, Ping L, Ngandu N, Karim QA, Karim SS, Swanstrom RI, Seaman MS, Williamson C, Morris L. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012 Nov;18(11):1688-92. doi: 10.1038/nm.2985. Epub 2012 Oct 21. PMID:23086475 doi:10.1038/nm.2985