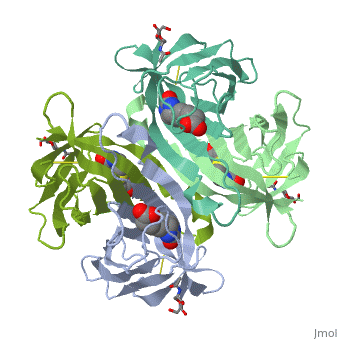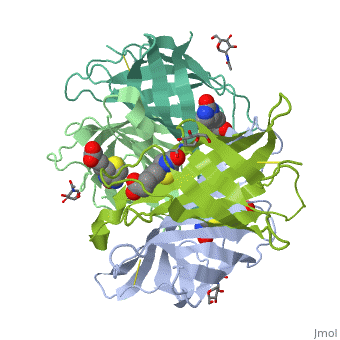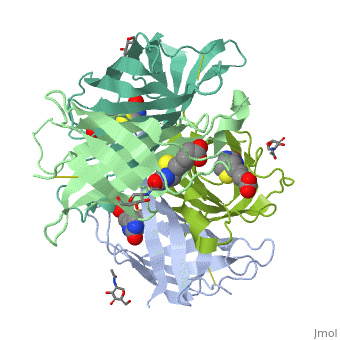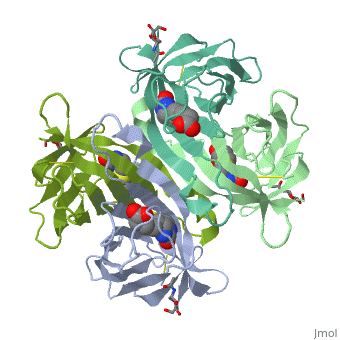User:Noam Gonen/Avidin
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | Intricate interaction, which is decisive to the observed structural stability of the avidin tetramer.The cohesion of the two monomers, is so intimate that it is difficult to distinguish between them in the resultant dimer. Sections of four β -strands (β4, β5, β6, and β7) from each monomer take part in this extensive interaction.The buried surface area of interaction is〖1951Å〗^2 . | ||
==Biotin binding == | ==Biotin binding == | ||
| + | '''Hydrophobic residues in the binding site of avidin''' | ||
| + | |||
| + | These include Trp-70, Phe-72, Phe-79, and Trp-97 from one monomer, and Trp-110 (dashed lines), which is provided by the adjacent symmetry-related monomer. | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 16:03, 17 January 2015
INTRODUCTION
Avidin is a tetrameric or dimeric[1] biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs.
| |||||||||||