Odorant binding protein
From Proteopedia
| Line 13: | Line 13: | ||
'''A few functions have been suggested for OBP:''' | '''A few functions have been suggested for OBP:''' | ||
| + | |||
1. Solubelizing the odorant molecule and its transportation in the sensillar lymph. | 1. Solubelizing the odorant molecule and its transportation in the sensillar lymph. | ||
| Line 35: | Line 36: | ||
====BmorPBP structure and function==== | ====BmorPBP structure and function==== | ||
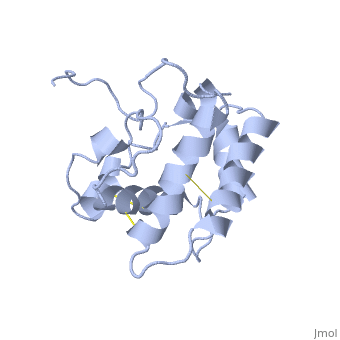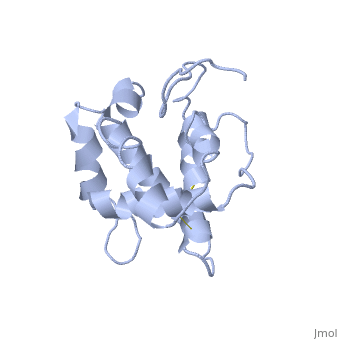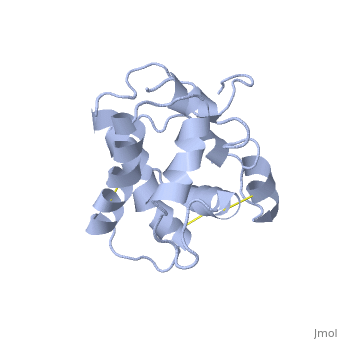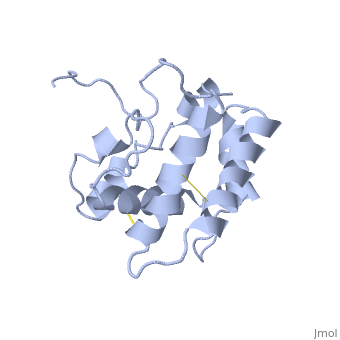
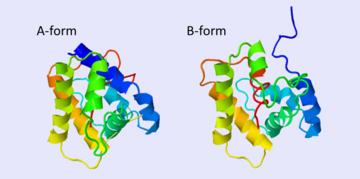
| - | The protein has 164 amino acids that forms 6-7 alpha helices. Three disulfide bonds (in yellow) formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust structure of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_residues/1'>charged residues</scene>, which allows it to make interactions with the water molecule and solubilize in the sensillar lymph. | + | The protein has 164 amino acids that forms 6-7 alpha helices (depends on the protein conformation). Three disulfide bonds (in yellow) formed by <scene name='68/683383/Cysteins6/1'>6 cystein </scene> residues tied four helices, and form the compact and robust structure of the protein. As expected from a soluble protein, its surface is covered with <scene name='68/683383/Charged_residues/1'>charged residues</scene>, which allows it to make interactions with the water molecule and solubilize in the sensillar lymph. |
| - | ====BmorPBP | + | ====BmorPBP - ligand binding==== |
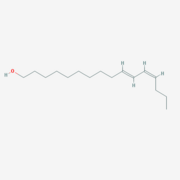
The protein natural ligand is the moth pheromone <scene name='68/683383/Bombykol_ligand_in_2p71/1'>Bombykol</scene>. However, it was demonstrated that other molecules can also bound to the protein cavity <ref>doi: 10.1016/j.str.2007.07.013</ref>. The interaction with the ligand is being made by 4 alpha helices 1, 4, 5 and 6 in the core of the protein, which form the binding cavity <ref>doi: 10.1016/S1074-5521(00)00078-8</ref>. | The protein natural ligand is the moth pheromone <scene name='68/683383/Bombykol_ligand_in_2p71/1'>Bombykol</scene>. However, it was demonstrated that other molecules can also bound to the protein cavity <ref>doi: 10.1016/j.str.2007.07.013</ref>. The interaction with the ligand is being made by 4 alpha helices 1, 4, 5 and 6 in the core of the protein, which form the binding cavity <ref>doi: 10.1016/S1074-5521(00)00078-8</ref>. | ||
Inside the binding cavity, <scene name='68/683383/Residues_interacting/1'>non-charged residues</scene> are interacting with the pheromone, mainly by van der waals bounds. Out of those residues, some are conserved across OBP of lepidopteran (<font color='green'><b>in green</b></font>), and the rest are conserved in lepidopteran PBP only (<font color='light blue'><b>in light blue</b></font>). | Inside the binding cavity, <scene name='68/683383/Residues_interacting/1'>non-charged residues</scene> are interacting with the pheromone, mainly by van der waals bounds. Out of those residues, some are conserved across OBP of lepidopteran (<font color='green'><b>in green</b></font>), and the rest are conserved in lepidopteran PBP only (<font color='light blue'><b>in light blue</b></font>). | ||
| Line 53: | Line 54: | ||
The c-terminus of the protein bears mostly non-polar amino acids. Yet on the surface of the helix there are three exceptional amino acids: Asp-132, Glu-137, and Glu-141, which are conserved in moth PBP <ref>doi: 10.1016/j.bbrc.2005.07.176</ref>. Of these, residues <scene name='68/683383/Bombykol_ligand_in_2p71/2'>Asp-132</scene> (and Glu-141, if present) triggers the formation of the alpha-helix upon protonation at low pH. This causes the <scene name='68/683383/1dqe_2fjy-bom/1'>ejaculation of the ligand from the binding pocket</scene>, which is replaced by the formatted alpha helix<ref>doi: 10.1016/j.bbrc</ref>. | The c-terminus of the protein bears mostly non-polar amino acids. Yet on the surface of the helix there are three exceptional amino acids: Asp-132, Glu-137, and Glu-141, which are conserved in moth PBP <ref>doi: 10.1016/j.bbrc.2005.07.176</ref>. Of these, residues <scene name='68/683383/Bombykol_ligand_in_2p71/2'>Asp-132</scene> (and Glu-141, if present) triggers the formation of the alpha-helix upon protonation at low pH. This causes the <scene name='68/683383/1dqe_2fjy-bom/1'>ejaculation of the ligand from the binding pocket</scene>, which is replaced by the formatted alpha helix<ref>doi: 10.1016/j.bbrc</ref>. | ||
| - | {{Button Toggle AnimationOnPause}} | ||
| - | |||
| - | <scene name='68/683383/1dqe_2fjy-bom_-1/1'>TextToBeDisplayed</scene> | ||
{{Button Toggle AnimationOnPause}} | {{Button Toggle AnimationOnPause}} | ||
| Line 70: | Line 68: | ||
*'''Activation by the complex pheromone-PBP''' | *'''Activation by the complex pheromone-PBP''' | ||
| - | An alternative mode of action was proposed for the receptor activation in | + | An alternative mode of action was proposed for the receptor activation in Drosophila, where it was found that the complex of pheromone-PBP is required for the activity of pheromone-sensitive neurons <ref>DOI: 10.1016/j.neuron.2004.12.031<ref/><ref>doi: 10.1016/j.cell.2008.04.046<ref/> |
</StructureSection> | </StructureSection> | ||
Revision as of 07:52, 19 January 2015
Contents |
Introduction
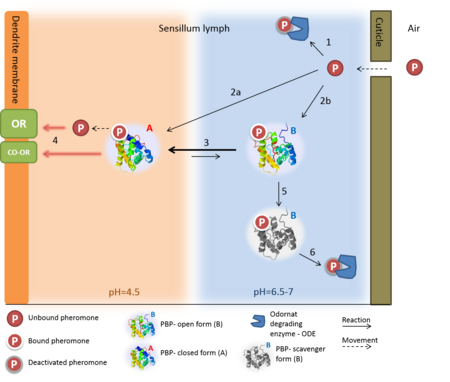
Odorant-binding protein (OBP) are soluble proteins which involve in the processes of odorant detection in the olfactory sensilla [1]
Though functionally same, vertebrates and insects OBP have different origin and structure. OBPs are important for insect olfaction. For instance, OBP76a (LUSH) in the fly Drosophila melanogaster is required for the detection of the pheromone vaccenyl acetate [2] and has been proven to adopt a conformation that activates the odorant receptor [3].

OBP in insects
OBP Function
Despite five decades of intensive research, the exact roles of OBP and the mechanism by which the odorant receptor (OR) is activated are still in dispute [4][5].
A few functions have been suggested for OBP:
1. Solubelizing the odorant molecule and its transportation in the sensillar lymph.
2. Protecting the odorant molecule from the odorant degrading enzymes, in the sensillar lymph.
3. Activating of the odorant receptor on the dendrite membrane, by the odorant-OBP complex.
4. Mediating the deactivation of the odorant molecule after the activation of the receptor.
5. An organic anion (the protein has 9 negative charges).
Of all, the first role of OBP as an odorant solubilizer and carrier is generally accepted.
In order to explain the structure and function of these fascinating proteins, this page will further focus on a particular OBP - the well investigated Bombyx mori PBP: BmorPBP.
Bombyx mori BmorPBP (lets talk about sex..)
| |||||||||||
See also
- Odorant_binding_protein_3D_structures
- Chemical communication in arthropods
- Pheromone binding protein
References
Proteopedia Page Contributors and Editors (what is this?)
Nurit Eliash, Michal Harel, Joel L. Sussman, Alexander Berchansky, Jaime Prilusky