Introduction
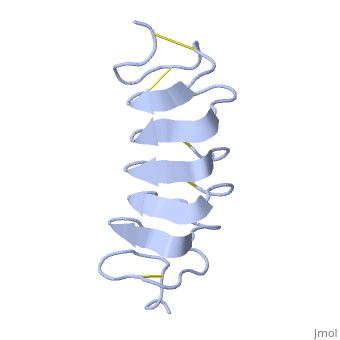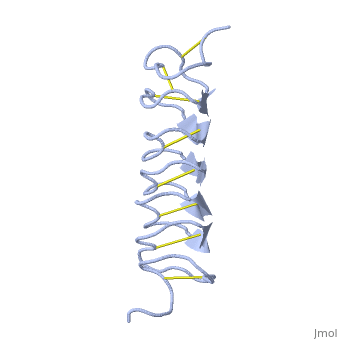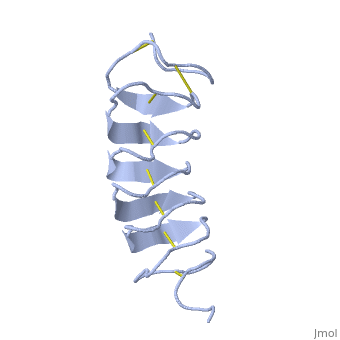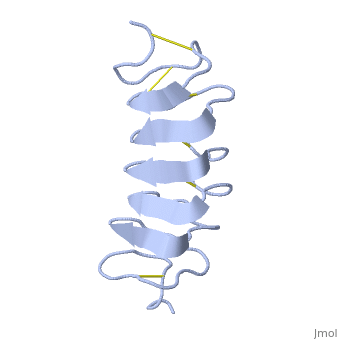
TmAFP is an hyperactive antifreeze protein and its origin is the Tenebrio Mollitor beetle (Mealworm). These beetles are found in dark and cold areas, the temperature range in which it can survive can reach very low degrees- for example- the polar climate. Due to TmAFP, Tenebrio Mollitor beetle has resistance against freezing - provides protection against physical and osmotic stresses. TmAFP is shaped like a slightly flattened cylinder 32Å in length, and 6.5X 14Å in the pseudo-rectangular cross section- one of the smallest Beta Hellix AFP known to date.
This is a view of the protein presenting the N terminal in blue and the C terminal in red.
Structure
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA.
TmAFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids (TCTxSxxCxxAx). , Theonine, Cystein, Threonine or ACT motifs are aligned to form a flat along one side of the molecule the Beta sheets right handed which are the binding site of the protein.
[1] The rest of the tandem repeat forms the loop which enables very organized structure of the protein. Cysteine all over the tandem repeats, are pared to provide the which contribute to the stability of the protein. Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other in the N-terminal region do not fit this pattern and involved in a capping structure. The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop connections between backbone CO and NH groups.
TmAFP is the one of smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. The few Hydrophobic residues have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.[2]
Ice binding site
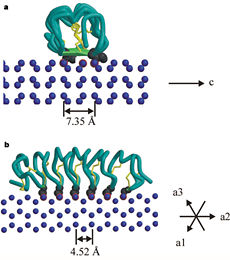
The two dimensional arrays of side chain makes a remarkably good match to the repeated spacing between oxygen atoms in the ice lattice on the primary prism plane, and a reasonable match to the basal plane, This is reason why the activity of TmAFP ( Thermal hysteresis) is much higher than the acticity of AFP from Fish (5-10 celcius degrees and 1.5 celcius degrees respectively)[3]
Lattice matching model for TmAFP binding to ice:
a- Prism plane.
b- Basal plane.
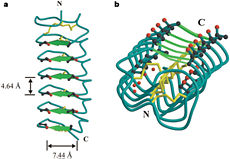
In solution the protein is monomeric and it can bind the ice just in this formation. The protein crystallized as a , the dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.
The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference.
[4]
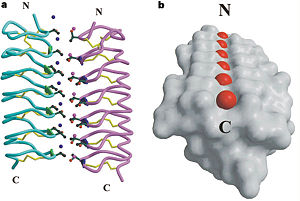
Dimer of TmAFP and organization of external water:
a- A TmAFP pair dimerized through hydrogen bonding to two ranks of ordered waters.
b- Surface presentation of TmAFP with the closest rank of water molecules.
Function
The function of the TmAFP (And other Antifreeze protein) is Thermal Hysteresis(TH) .TH activity is occurs due to an adsorption inhibition mechanism that’s states that AFPs binds to ice surface and allow ice crystal growths only in surface regions between the bound AFP. Growing curvature causes an increase in surface energy, making the transformation of water into ice lass energetically favorable. Because of that the AFPs lower freezing temperature below the melting point. The difference between the melting and freezing point of the ice called thermal hysteresis activity.
The difference between TmAFP (hyperactive AFP) and Type I AFP (moderate AFP)
TmAFP has special structure of beta helix, due to it the binding site consist of two dimensional surface, two arrays of Threonine residues that can bind to the two plans of ice, prism plane and basal plan. This is why the TH activity can reach 6 Celsius degrees (Hyperactive protein).
In contrast , from the fish winter flounder (moderate AFP are usually fish origin), structure is alpha helix allows just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1.5 Celsius degree.
Antifreeze protein