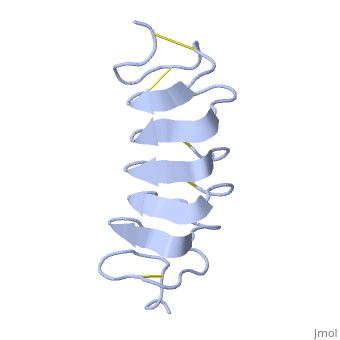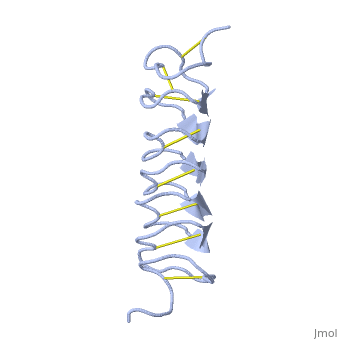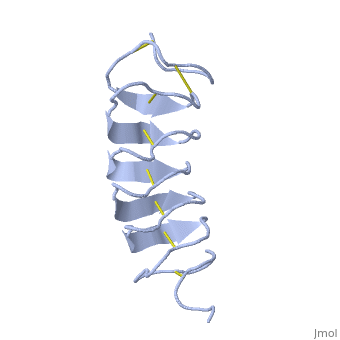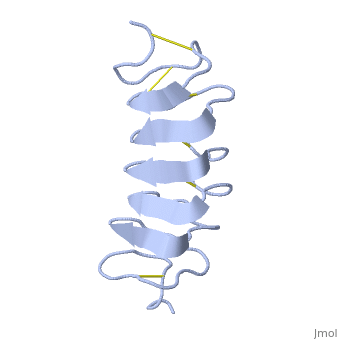
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
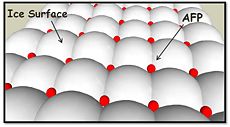
Antifreeze proteins (AFPs) produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind TO ice crystals to inhibit growth of ice that would otherwise be fatal to those orgnisms. | Antifreeze proteins (AFPs) produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind TO ice crystals to inhibit growth of ice that would otherwise be fatal to those orgnisms. | ||
AFPs are classified to two groups on the basis of their activity (TH), moderate and hyperactive AFPs. | AFPs are classified to two groups on the basis of their activity (TH), moderate and hyperactive AFPs. | ||
| - | Modrete AFP | + | Modrete AFP are usually fish origin |
| - | Hyperactive AFP | + | Hyperactive AFP are usually insacts origin but recently have been discovered in other organisms including bacteria.<ref>DOI doi:10.1016/j.cryobiol.2006.06.006</ref> |
| Line 52: | Line 52: | ||
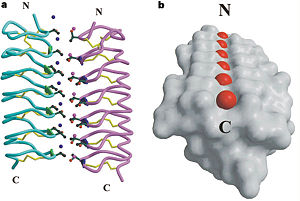
== The difference between TmAFP (hyperactive AFP) and Type I AFP (moderate AFP) == | == The difference between TmAFP (hyperactive AFP) and Type I AFP (moderate AFP) == | ||
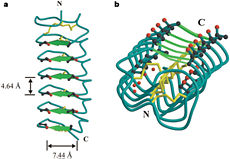
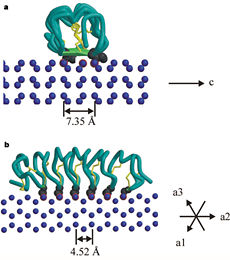
| - | TmAFP has special structure of beta helix, due to it the binding site consist of two dimensional surface, two arrays of Threonine residues <scene name='61/612804/Thr/2'>two arrays of Threonine residues</scene> that can bind to the two plans of ice, prism plane and basal plan. This is why the TH activity can reach 6 Celsius degrees | + | TmAFP has special structure of beta helix, due to it the binding site consist of two dimensional surface, two arrays of Threonine residues <scene name='61/612804/Thr/2'>two arrays of Threonine residues</scene> that can bind to the two plans of ice, prism plane and basal plan. This is why the TH activity can reach 6 Celsius degrees in Hyperactive protein, tmAFP can reach 1.5 Celsius degree. |
| - | In contrast <scene name='61/612804/Afp1/1'>Type I AFP</scene>, from the fish winter flounder (moderate AFP are usually fish origin), structure is alpha helix allows just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1 | + | In contrast <scene name='61/612804/Afp1/1'>Type I AFP</scene>, from the fish winter flounder (moderate AFP are usually fish origin), structure is alpha helix allows just one dimensional surface of threonine residue and thus can bind only to one plane of ice (the prism plan). Because of that The TH activity is much lower, maximum TH activity of moderate AFP is 1 Celsius degree. |
[[Antifreeze protein]] | [[Antifreeze protein]] | ||
Revision as of 16:35, 19 January 2015
| |||||||||||
References
- ↑ Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL. The basis for hyperactivity of antifreeze proteins. Cryobiology. 2006 Oct;53(2):229-39. Epub 2006 Aug 2. PMID:16887111 doi:http://dx.doi.org/10.1016/j.cryobiol.2006.06.006
- ↑ Liu K, Jia Z, Chen G, Tung C, Liu R. Systematic size study of an insect antifreeze protein and its interaction with ice. Biophys J. 2005 Feb;88(2):953-8. PMID:15713600 doi:http://dx.doi.org/10.1529/biophysj.104.051169
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ doi: https://dx.doi.org/10.1016/S0968-0004(01)02028-X
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604