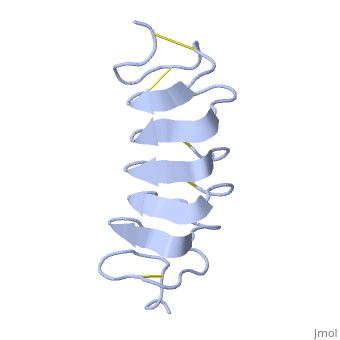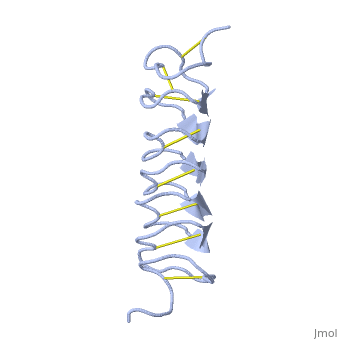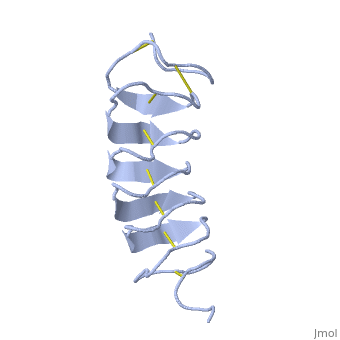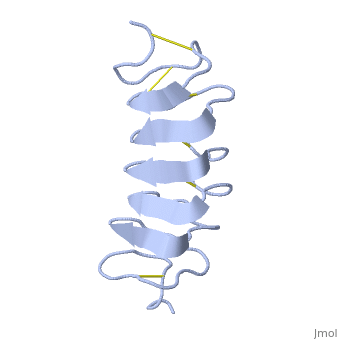
Tenebrio molitor Antifreeze Protein (TmAFP)
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | The protein consists of 84 amino acids and the molecular weight is 8.4 kDA. | ||
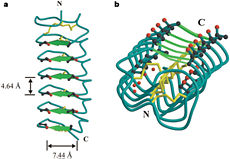
| - | ''Tm''AFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids (TCTxSxxCxxAx). <scene name='61/612804/Tct/1'>TCT </scene>, Theonine, Cystein, Threonine or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein.<ref>DOI 10.1529/biophysj.104.051169</ref> The rest of the tandem repeat forms the loop which enables very organized structure of the protein. Cysteine all over the tandem repeats, are pared to provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> in the N-terminal region do not fit this pattern and involved in a capping structure. The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop <scene name='61/612804/Hbond/2'>hydrogen bond</scene> connections between backbone CO and NH groups.[[Image:406322aa.2.jpg|230px|right]] | + | ''Tm''AFP is protein rich of threonine and cystein in form of regular parallel beta-helix. It composed of 7 tandem repeats which consist of 12 amino acids (TCTxSxxCxxAx). <scene name='61/612804/Tct/1'>TCT </scene>, Theonine, Cystein, Threonine or ACT motifs are aligned to form a flat <scene name='61/612804/Beta_sheet/1'>Beta sheet</scene> along one side of the molecule the Beta sheets right handed which are the binding site of the protein.<ref name="one">DOI 10.1529/biophysj.104.051169</ref> The rest of the tandem repeat forms the loop which enables very organized structure of the protein. Cysteine all over the tandem repeats, are pared to provide the <scene name='61/612804/Cys/1'>disulphid bonds</scene> which contribute to the stability of the protein. Six of the eight disulphide bounds construct near perfect alignment enables appropriate structure that allows binding to the ice lattice. The other <scene name='61/612804/2disulphide/1'>two disulphide bonds</scene> in the N-terminal region do not fit this pattern and involved in a capping structure. The extraordinary tightness of the 12 amino-acids turn is also facilitated by intra-loop <scene name='61/612804/Hbond/2'>hydrogen bond</scene> connections between backbone CO and NH groups.[[Image:406322aa.2.jpg|230px|right]] |
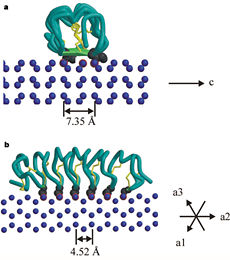
''Tm''AFP is the one of smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. The few Hydrophobic residues <scene name='61/612804/Hydrophobic/2'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.<ref>DOI 10.1038/35018604</ref> | ''Tm''AFP is the one of smallest Beta-Helix with only 12 amino acids per turn. Therefore, it has a very narrow bore, which is constricted and further bisected by disulphide bonds to form two channels, leaving no room for hydrophobic core. The few Hydrophobic residues <scene name='61/612804/Hydrophobic/2'>val25, val34, phe58, tyr70</scene> have their side chains projecting outwards to the solvent. In the core there is room only for the relatively small side chains of the conserves Serine and Alanine to project into the core, on either side of the bisecting disulphide bridge.<ref>DOI 10.1038/35018604</ref> | ||
| Line 36: | Line 36: | ||
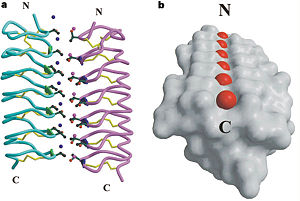
| - | In solution the protein is monomeric and it can bind the ice just in this formation. The protein crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>, the dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.[[Image:406322ac.2.jpg|300px|right]] The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference. <ref | + | In solution the protein is monomeric and it can bind the ice just in this formation. The protein crystallized as a <scene name='61/612804/Dimer/1'>dimer</scene>, the dimerization occurs along the surface of the beta sheets. The 2 units of the dimer do not directly interact with each other, the contact between them is mediated by highly ordered ranks of water which form hydrogen bonding with Threonine residue. Each water molecule forms two hydrogen bonds to the closer monomer and one to the distant monomer. The distance between two adjacent waters is 4.64±0.20Å the same distance as between Threonine residues 4.64±0.23Å. A good two dimensional match to the ice lattice including all 3 ranks of oxygen atoms (2 from Thr and 1 from water), implying that the ordered water molecules cold act as part of an ice surface and directly participate in the AFP-ice interaction.[[Image:406322ac.2.jpg|300px|right]] The regular array formed by this 3 ranks of oxygen atoms can be seen as a small piece of one layer thick ice to be incorporated into a large ice lattice. There is no need to readjust Threonine side chains, because they don’t present steric interference. <ref name="one"> |
'''Dimer of TmAFP and organization of external water:''' | '''Dimer of TmAFP and organization of external water:''' | ||
Revision as of 16:43, 19 January 2015
| |||||||||||
References
- ↑ Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL. The basis for hyperactivity of antifreeze proteins. Cryobiology. 2006 Oct;53(2):229-39. Epub 2006 Aug 2. PMID:16887111 doi:http://dx.doi.org/10.1016/j.cryobiol.2006.06.006
- ↑ 2.0 2.1 Liu K, Jia Z, Chen G, Tung C, Liu R. Systematic size study of an insect antifreeze protein and its interaction with ice. Biophys J. 2005 Feb;88(2):953-8. PMID:15713600 doi:http://dx.doi.org/10.1529/biophysj.104.051169
- ↑ Liou YC, Tocilj A, Davies PL, Jia Z. Mimicry of ice structure by surface hydroxyls and water of a beta-helix antifreeze protein. Nature. 2000 Jul 20;406(6793):322-4. PMID:10917536 doi:10.1038/35018604
- ↑ doi: https://dx.doi.org/10.1016/S0968-0004(01)02028-X