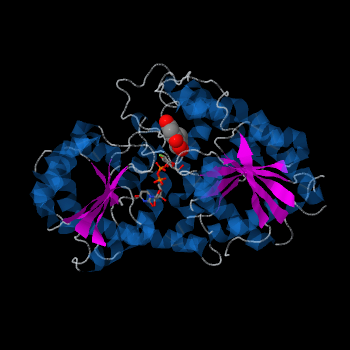
Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT)
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
=== GT-B === | === GT-B === | ||
| - | Plant glycosyltransferases assume one of two folds, GT-A or GT-B. Vv3GT is a GT-B enzyme.<ref>PMID:18829982</ref> GT-B enzymes consists of two β/α/β Rossmann-like domains. The two domains are associated and face each other with the active-site lying between them. These domains are associated with the donor and acceptor substrate binding sites. | + | Plant glycosyltransferases assume one of two folds, GT-A or GT-B. Vv3GT is a GT-B enzyme.<ref>PMID:18829982</ref> GT-B enzymes consists of two β/α/β Rossmann-like domains <ref>PMID:16482224</ref>. The two domains are associated and face each other with the active-site lying between them. These domains are associated with the donor and acceptor substrate binding sites. |
The <scene name='69/692252/2c1z_rainbow/1'>N-terminal and C-terminal domain</scene>, linked by a flexible loop and α-helix with a hinge region. The linker could be important for sugar donor binding. This linker region holds the UDP-sugar donor next to the C-terminal PSPG motif. The C-terminal domain is highly conserved and binds the UDP sugar donor (e. g. UDP-Glc). On the other hand, the N-terminal domain is not conserved it binds the substrate and provides catalytically active amino acids. | The <scene name='69/692252/2c1z_rainbow/1'>N-terminal and C-terminal domain</scene>, linked by a flexible loop and α-helix with a hinge region. The linker could be important for sugar donor binding. This linker region holds the UDP-sugar donor next to the C-terminal PSPG motif. The C-terminal domain is highly conserved and binds the UDP sugar donor (e. g. UDP-Glc). On the other hand, the N-terminal domain is not conserved it binds the substrate and provides catalytically active amino acids. | ||
Revision as of 10:32, 25 January 2015
| |||||||||||
References
- ↑ Frydman A, Weisshaus O, Bar-Peled M, Huhman DV, Sumner LW, Marin FR, Lewinsohn E, Fluhr R, Gressel J, Eyal Y. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1,2RhaT encoding a 1,2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 2004 Oct;40(1):88-100. PMID:15361143 doi:http://dx.doi.org/10.1111/j.1365-313X.2004.02193.x
- ↑ Osmani SA, Bak S, Imberty A, Olsen CE, Moller BL. Catalytic key amino acids and UDP-sugar donor specificity of a plant glucuronosyltransferase, UGT94B1: molecular modeling substantiated by site-specific mutagenesis and biochemical analyses. Plant Physiol. 2008 Nov;148(3):1295-308. doi: 10.1104/pp.108.128256. Epub 2008, Oct 1. PMID:18829982 doi:http://dx.doi.org/10.1104/pp.108.128256
- ↑ Osmani SA, Bak S, Imberty A, Olsen CE, Moller BL. Catalytic key amino acids and UDP-sugar donor specificity of a plant glucuronosyltransferase, UGT94B1: molecular modeling substantiated by site-specific mutagenesis and biochemical analyses. Plant Physiol. 2008 Nov;148(3):1295-308. doi: 10.1104/pp.108.128256. Epub 2008, Oct 1. PMID:18829982 doi:http://dx.doi.org/10.1104/pp.108.128256
- ↑ Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006 Mar 22;25(6):1396-405. Epub 2006 Feb 16. PMID:16482224