We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
RiAFP
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
== Ice Binding Surface (IBS) == | == Ice Binding Surface (IBS) == | ||
[[Image:Fig4_D.jpg|frame|left|Fig. 2. Ice-binding surface ]] | [[Image:Fig4_D.jpg|frame|left|Fig. 2. Ice-binding surface ]] | ||
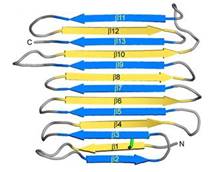
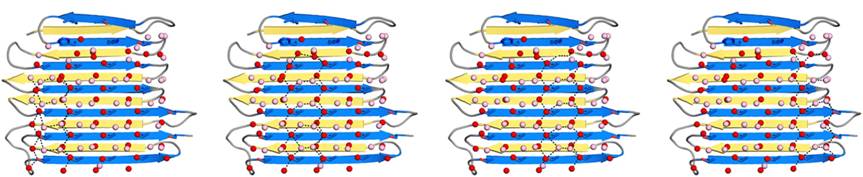
| - | IBS of RiAFP contains five expanded <scene name='60/607864/Ibs/1'>TXTXTXT motifs</scene> within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTTX motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the | + | IBS of RiAFP contains five expanded <scene name='60/607864/Ibs/1'>TXTXTXT motifs</scene> within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTTX motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the TH. The Thr hydroxyls define a large flat IBS of 420 Å2, which correlates with high antifreeze activity (Figure 2). |
| Line 24: | Line 24: | ||
== Molecular Basis for Ice Binding == | == Molecular Basis for Ice Binding == | ||
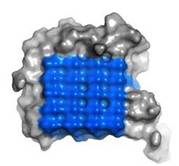
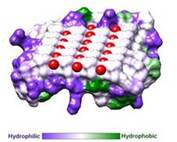
| - | IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice ( | + | IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice (Figure 3). Adsorption of the AFP ice-binding surface to ice is facilitated by the flatness of the IBS of the ''Ri''AFP. The Sc (shape complementarity) values between ''Ri''AFP and ice interfaces range from 0.75-0.78, where 1.0 indicates perfect match. For comparison, antigen-antibody complexes usually have their Sc values in the range of 0.64–0.68. |
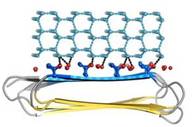
| - | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/3'>water molecules</scene> with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the simulation appear to be organized in an ice-like formation, with close matches to the primary prism and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice ( | + | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/3'>water molecules</scene> with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the computational simulation appear to be organized in an ice-like formation, with close matches to the primary prism and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice (Figure 4). |
{|style="margin: 0 auto;" | {|style="margin: 0 auto;" | ||
| Line 33: | Line 33: | ||
| - | An advantage of this indirect binding mechanism is that the organized waters are still fluid enough to make flexible matches to the ice-like quasi-liquid layer around the ice before becoming rigidified as the junction layer freezes. Alignment of IBS of | + | An advantage of this indirect binding mechanism is that the organized waters are still fluid enough to make flexible matches to the ice-like quasi-liquid layer around the ice before becoming rigidified as the junction layer freezes. Alignment of IBS of ''Ri''AFP to hexagonal ice showed possible interactions of the protein with the several ice planes. Several ranks of matching Thr hydroxyls to water molecules give the ''Ri''AFP an ability to bind multiple ice planes, by that providing the protein hyperactivity (Figure 5). |
Revision as of 17:12, 25 January 2015
| |||||||||||
3D structures of antifreeze protein
References
- ↑ Jia Z, Davies PL. Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci. 2002 Feb;27(2):101-6. PMID:11852248
- ↑ Chantelle J. Capicciotti, Malay Doshi and Robert N. Ben (2013). Ice Recrystallization Inhibitors: From Biological Antifreezes to Small Molecules, Recent Developments in the Study of Recrystallization, Prof. Peter Wilson (Ed.), ISBN: 978-953-51-0962-4, InTech doi:http://dx.doi.org/10.5772/54992
- ↑ Drori R, Celik Y, Davies PL, Braslavsky I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J R Soc Interface. 2014 Sep 6;11(98):20140526. doi: 10.1098/rsif.2014.0526. PMID:25008081 doi:http://dx.doi.org/10.1098/rsif.2014.0526
- ↑ Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589-93. PMID:267952
- ↑ Hakim A, Nguyen JB, Basu K, Zhu DF, Thakral D, Davies PL, Isaacs FJ, Modis Y, Meng W. Crystal structure of an insect antifreeze protein and its implications for ice binding. J Biol Chem. 2013 Apr 26;288(17):12295-304. doi: 10.1074/jbc.M113.450973. Epub, 2013 Mar 12. PMID:23486477 doi:10.1074/jbc.M113.450973