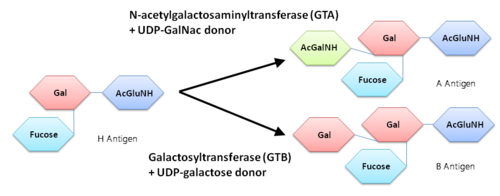
Human ABO(H) Blood Group Glycosyltransferases
From Proteopedia
(Difference between revisions)
| Line 56: | Line 56: | ||
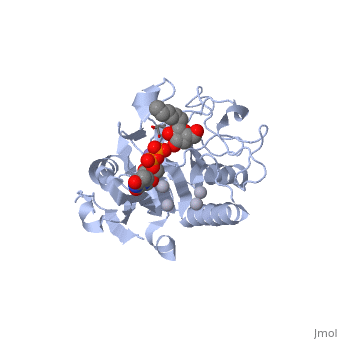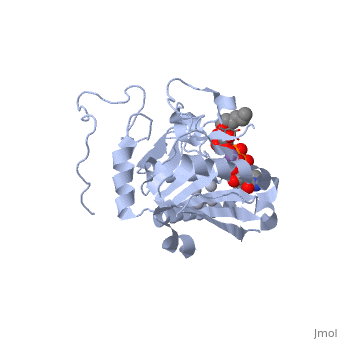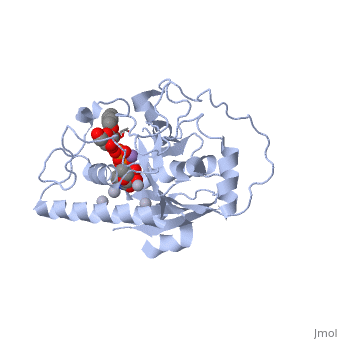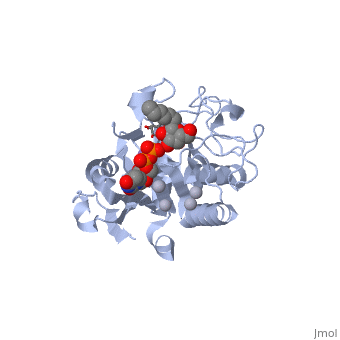
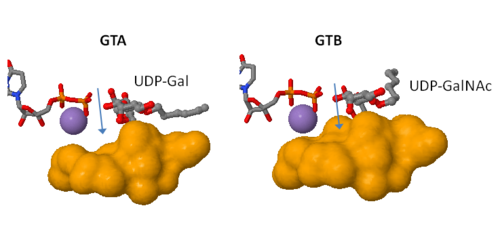
only Leu/Met 266 is positioned to contact the characteristic acetamido/hydroxyl groups and so distinguishes between UDP-GalNAc and UDP-Gal. This is a complementary interaction where the larger acetamido group in UDP-GalNAc is accommodated by the smaller Leu 266 in GTA, whereas the corresponding smaller hydroxyl group on UDP-Gal is accommodated by the larger Met 266 in GTB. | only Leu/Met 266 is positioned to contact the characteristic acetamido/hydroxyl groups and so distinguishes between UDP-GalNAc and UDP-Gal. This is a complementary interaction where the larger acetamido group in UDP-GalNAc is accommodated by the smaller Leu 266 in GTA, whereas the corresponding smaller hydroxyl group on UDP-Gal is accommodated by the larger Met 266 in GTB. | ||
| + | [[Image:Floor.png|500px|right]] | ||
Residues Met 266 and Ala 268 in GTB are both bulkier than the corresponding residues in GTA, with the result that the floor of the active site is raised in GTB with respect to GTA. This provides GTB with a significantly smaller and, therefore, more conformationally restricted active site cleft, such that these residues serve to exclude UDP-GalNAc and position UDP-Gal. In GTA, the floor of the active site is lower, providing a much larger active site cleft that allows a high degree of motion to a potential UDP-Gal substrate; that is, the active site cleft in GTA is essentially a large void to UDP-Gal where it has little possibility of forming the complementary interactions that are required for substrate recognition and enzyme action. In addition, the lowered floor of the active site cleft in GTA reveals His 233, which can form a hydrogen bond with the acetamido group of UDP-GalNAc and allow the sugar residue to be selectively positioned. | Residues Met 266 and Ala 268 in GTB are both bulkier than the corresponding residues in GTA, with the result that the floor of the active site is raised in GTB with respect to GTA. This provides GTB with a significantly smaller and, therefore, more conformationally restricted active site cleft, such that these residues serve to exclude UDP-GalNAc and position UDP-Gal. In GTA, the floor of the active site is lower, providing a much larger active site cleft that allows a high degree of motion to a potential UDP-Gal substrate; that is, the active site cleft in GTA is essentially a large void to UDP-Gal where it has little possibility of forming the complementary interactions that are required for substrate recognition and enzyme action. In addition, the lowered floor of the active site cleft in GTA reveals His 233, which can form a hydrogen bond with the acetamido group of UDP-GalNAc and allow the sugar residue to be selectively positioned. | ||
Revision as of 01:24, 26 January 2015
| |||||||||||