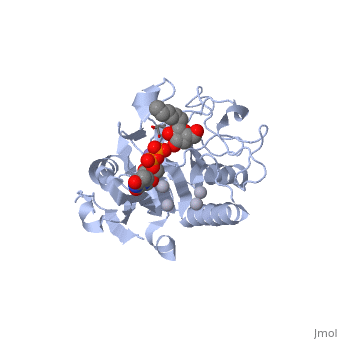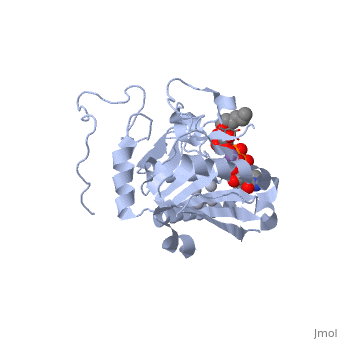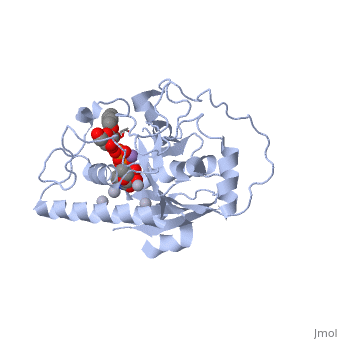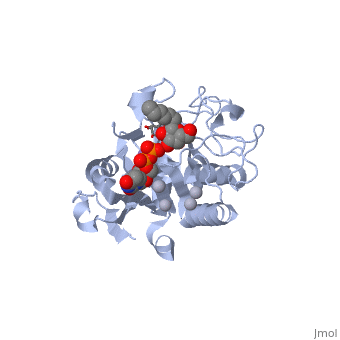
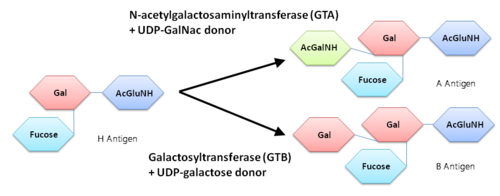
Human ABO(H) blood group glycosyltransferases GTA and GTB catalyze the final monosaccharide addition in the biosynthesis of the human A and B blood group antigens.
Introduction
We can categorize our blood types into 4 groups: A, B, AB, & O, on the surface of red blood cells, there are 2 types of antigen: A & B (each type has its own properties) If a blood cell is type A, the surface of the cell contains Antigens for type A and the body will produce antibodies for type B and vice versa for type B. Type AB contains both antigens on the surface and has neither antibodies. Blood type O has no antigens and thus have both A and B antibodies in its system.
The A and the B antigens were found to be modified from the carbohydrate corresponding to the O blood group by the addition of different monosaccharides, the A antigen was terminated by the sugar N-acetylgalactosmine (GalNAc) while the B antigen was terminated by galactose.
The human ABO(H) blood group antigens are produced by specific glucosyltransferase enzymes (GTs), the ABO blood group system is determined by what type of glucosyltransferases are expressed in the body: N-acetylgalactosaminyltransferase or Galactosyltransferase .
Reaction
An N-acetylgalactosaminyltrasferase (GTA) uses a UDP-GalNac donor to convert the H-antigen acceptor to the A antigen, whereas a galactosyltransferase (GTB) uses UDP-galactose donor to convert the H-antigen acceptor to the B antigen.
Genetics
The ABO gene locus expressing the glycosyltransferases has three main alleleic forms: A, B, and O, the A allele encodes GTA. The B allele encodes GTB, the O allele differs slightly from the A allele by deletion of a single nucleotide. The deletion causes a frameshift and results in translation of an almost entirely different protein that lacks enzymatic activity. This results in H antigen remaining unchanged in case of O groups.
Structural highlights
General topology of GTA and GTB
Both GTs consist of 354 residues and they only differ by four "critical" amino acids[1].
the polypeptide chain is organized in separated by a cleft, approximately 13 Å wide, containing the active site which consist all four critical amino acid residues. The N-terminal (blue), includes a Rossmann fold and recognizes the nucleotide donor, whereas the disaccharide acceptor binding site is formed by residues in the C-terminal domain (green) in combination with bound UDP.
In the middle of the cleft located a , highly conserved in a large number of glycosyltransferases, which coordinates the Mn2+ ion and was suggested to have a role in catalysis[2].
Studies show that binding of the donor must precede acceptor binding. Indeed, the donor is a key preliminary step in the formation of the acceptor-binding site as both sugar residues of the acceptor make strong hydrogen bonds with the beta-phosphate group of the UDP.
The donor binds to the enzyme through Phe 121, Ile 123, Tyr 126 and Asp 213.
The H-antigen acceptor interacts with the enzyme primarily through the galactose O4-hydroxyl group, making hydrogen bond interactions with the side chains of His 233 and Glu 303, and the Gal O6-hydroxyl group, which hydrogen bonds to Thr 245 and the Fuc O4'-hydroxyl to Asp 326.
Structural basis for specifity
Arg/Gly 176
There are three conformations for the GTs enzymes, "open", "semi closed" and "closed" as they go from unliganded to the liganded states.
the open conformation for the enzyme, in the absence of donor or acceptor, the nine C-terminal residues are disordered and a major portion of the internal loop (amino acid residues 177-195) is disordered, the semi closed and closed forms of the enzymes are generated by binding of UDP or of UDP and H antigen, respectively. The closed form is distinguished from the semiclosed form by the ordering of the final nine C-terminal residues through the formation of hydrogen bonds to both UDP and H antigen. The semi-closed forms for various mutants generally show significantly more disorder than the open forms, whereas the closed forms display little or no disorder depending strongly on the identity of residue 176.
The side chain of the amino acid residue itself does not make any contact with any other part of the enzyme in any structure but greatly influences the level of flexibility in both the internal loop and the C-terminal region.
Gly/Ser 235
It is also not positioned to directly contact the donor, although this residue forces the aliphatic tail on the acceptor to assume different conformations in the and .
Out of the four critical amino acids only two can make contact with the donor.
Gly/Ala 268
Gly/Ala 268 can contact only moieties of the donor sugar residues that are identical in UDP-GalNAc and UDP-Gal (specifically hydroxyl groups O3 and O4), which does not explain how this residue could contribute to substrate specificity.
Therefore, the ability of the two enzymes to distinguish between the A and B donors is largely determined by a single amino acid residue.
Leu/Met 266
only Leu/Met 266 is positioned to contact the characteristic acetamido/hydroxyl groups and so distinguishes between UDP-GalNAc and UDP-Gal. This is a complementary interaction where the larger acetamido group in UDP-GalNAc is accommodated by the smaller Leu 266 in GTA, whereas the corresponding smaller hydroxyl group on UDP-Gal is accommodated by the larger Met 266 in GTB.
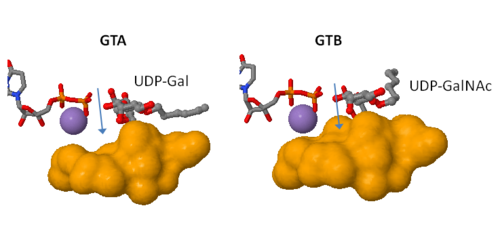
Residues Met 266 and Ala 268 in GTB are both bulkier than the corresponding residues in GTA, with the result that the floor of the active site is raised in GTB with respect to GTA. This provides GTB with a significantly smaller and, therefore, more conformationally restricted active site cleft, such that these residues serve to exclude UDP-GalNAc and position UDP-Gal. In GTA, the floor of the active site is lower, providing a much larger active site cleft that allows a high degree of motion to a potential UDP-Gal substrate; that is, the active site cleft in GTA is essentially a large void to UDP-Gal where it has little possibility of forming the complementary interactions that are required for substrate recognition and enzyme action. In addition, the lowered floor of the active site cleft in GTA reveals His 233, which can form a hydrogen bond with the acetamido group of UDP-GalNAc and allow the sugar residue to be selectively positioned.