We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Tachyplesin
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
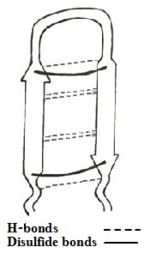
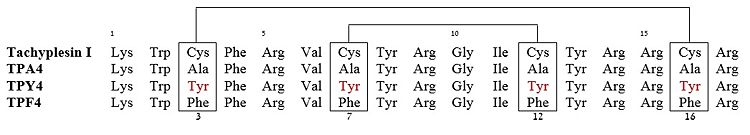
The β-hairpin topology of CDT is sustained by the <scene name='67/671725/Cdthaipin/2'>unique packing interactions</scene> between the aromatic ring of Trp2 and the side-chain of nonpolar amino acid of Val5 and the cationic side-chain of residue Arg11. | The β-hairpin topology of CDT is sustained by the <scene name='67/671725/Cdthaipin/2'>unique packing interactions</scene> between the aromatic ring of Trp2 and the side-chain of nonpolar amino acid of Val5 and the cationic side-chain of residue Arg11. | ||
Also, there exists close proximity between residues <scene name='67/671725/Cdtnoe/1'>Trp2 and Ile9</scene> which is supported by [http://en.wikipedia.org/wiki/Nuclear_Overhauser_effect nuclear overhauser effects (NOEs)] involving [http://en.wikipedia.org/wiki/Indole indole] ring protons of Trp2 with side-chain proton of Ile9. These packing interactions have rendered an approximate anti-parallel orientation of the hairpin structure of CDT in presence of LPS. | Also, there exists close proximity between residues <scene name='67/671725/Cdtnoe/1'>Trp2 and Ile9</scene> which is supported by [http://en.wikipedia.org/wiki/Nuclear_Overhauser_effect nuclear overhauser effects (NOEs)] involving [http://en.wikipedia.org/wiki/Indole indole] ring protons of Trp2 with side-chain proton of Ile9. These packing interactions have rendered an approximate anti-parallel orientation of the hairpin structure of CDT in presence of LPS. | ||
| - | The β-hairpin structure displays an extended <font color='darkblue'>positively charged</font> surface patch of <scene name='67/671725/Cdtr4r7r12r13/3'> | + | The β-hairpin structure displays an extended <font color='darkblue'>positively charged</font> surface patch of residues <scene name='67/671725/Cdtr4r7r12r13/3'>Arg 4, 7, 12 and 13</scene>. These positively charged basic residues interacts with the anionic phosphate groups of LPS, that leads to a plausible disruption or fluidization of LPS structures. This facilitates traversal of the peptide through the LPS-outer membrane<ref name=Saravanan>PMID:22464970</ref>. |
== Mode of action == | == Mode of action == | ||
Revision as of 06:27, 26 January 2015
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Laederach A, Andreotti AH, Fulton DB. Solution and micelle-bound structures of tachyplesin I and its active aromatic linear derivatives. Biochemistry. 2002 Oct 15;41(41):12359-68. PMID:12369825
- ↑ 2.0 2.1 Chen, Yixin, et al. "RGD-Tachyplesin inhibits tumor growth." Cancer research 61.6 (2001): 2434-2438.
- ↑ 3.0 3.1 Saravanan R, Mohanram H, Joshi M, Domadia PN, Torres J, Ruedl C, Bhattacharjya S. Structure, activity and interactions of the cysteine deleted analog of tachyplesin-1 with lipopolysaccharide micelle: Mechanistic insights into outer-membrane permeabilization and endotoxin neutralization. Biochim Biophys Acta. 2012 Mar 23;1818(7):1613-1624. PMID:22464970 doi:10.1016/j.bbamem.2012.03.015
- ↑ Nakamura, Takanori, et al. "Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure." Journal of Biological Chemistry 263.32 (1988): 16709-16713
- ↑ 5.0 5.1 Kushibiki T, Kamiya M, Aizawa T, Kumaki Y, Kikukawa T, Mizuguchi M, Demura M, Kawabata SI, Kawano K. Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim Biophys Acta. 2014 Jan 2;1844(3):527-534. doi:, 10.1016/j.bbapap.2013.12.017. PMID:24389234 doi:http://dx.doi.org/10.1016/j.bbapap.2013.12.017
- ↑ 6.0 6.1 6.2 Hong, Jun, et al. "Mechanism of Tachyplesin I injury to bacterial membranes and intracellular enzymes, determined by laser confocal scanning microscopy and flow cytometry." Microbiological research (2014)
- ↑ Yonezawa A, Kuwahara J, Fujii N, Sugiura Y. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry. 1992 Mar 24;31(11):2998-3004. PMID:1372516
- ↑ Lipsky A, Cohen A, Ion A, Yedidia I. Genetic transformation of Ornithogalum via particle bombardment and generation of Pectobacterium carotovorum-resistant plants. Plant Sci. 2014 Nov;228:150-8. doi: 10.1016/j.plantsci.2014.02.002. Epub 2014 Feb, 12. PMID:25438795 doi:http://dx.doi.org/10.1016/j.plantsci.2014.02.002
Proteopedia Page Contributors and Editors (what is this?)
Shulamit Idzikowski, Janak Raj Joshi, Michal Harel, Alexander Berchansky, Joel L. Sussman, Angel Herraez, Jaime Prilusky