We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ubc9
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
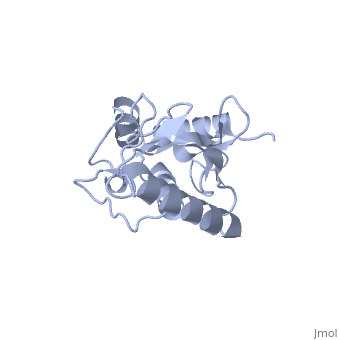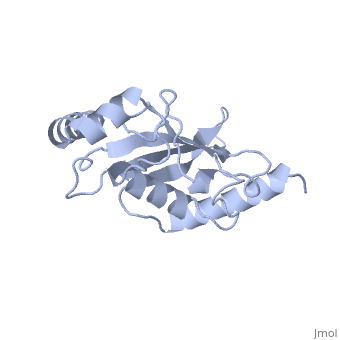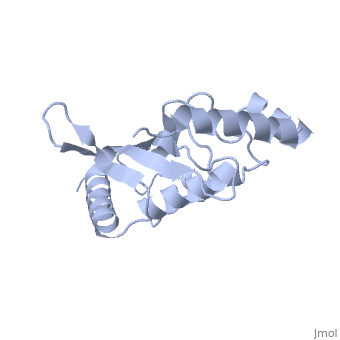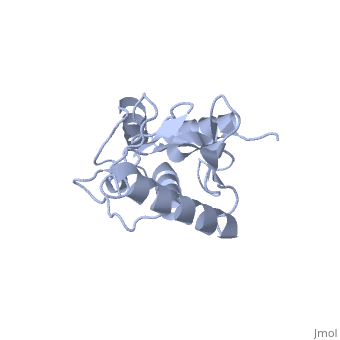
<StructureSection load='1U9A' size='350' side='right' caption='Human Ubiquitin Conjugating Protein Ubc9 ' scene=''> | <StructureSection load='1U9A' size='350' side='right' caption='Human Ubiquitin Conjugating Protein Ubc9 ' scene=''> | ||
| - | ''Ubc9'' is a [[ubiquitin conjugating enzyme]] (E2) whose function involves transfer of [[ubiquitin]] or [[small ubiquitin-like modifier]] (SUMO) from [[ubiquitin activating enzyme]] (E1) to its designated substrate. Ubc9 is specifically involved in SUMO transfer <ref name="crystal">PMID:9261152</ref>. | + | ''Ubc9'' is a [[ubiquitin conjugating enzyme]] (E2) whose function involves transfer of [[ubiquitin]] or [[small ubiquitin-like modifier]] (SUMO) from [[ubiquitin activating enzyme]] (E1) to its designated substrate. Ubc9 is specifically involved in SUMO transfer<ref name="crystal">PMID:9261152</ref>. |
== Structure == | == Structure == | ||
| Line 10: | Line 10: | ||
== Function == | == Function == | ||
| - | ''Ubc9'' is a part of the SUMOylation process. It is the enzyme responsible for ligating the SUMO to the protein. Depending on whether the reaction is done ''in vitro'', it will ligate the SUMO directly to the substrate, and if done ''in vivo'', the SUMO will be ligated to the conjugating enzyme and then put onto the substrate [2]. Mutagenesis studies on ''Ubc9'' SUMO1 complex have shown that ''Ubc9H20D'' and ''SUMO1E67R'' mutation cause inhibition of noncovalent interaction between the two, and thus inhibit complex formation. A similar interaction is seen in the ''Ubc9''-SUMO2 complex <ref name="ubcsumocomplex"/>. Lack of noncovalent interaction between ''Ubc9'' and SUMO2 has been shown to inhibit SUMO2 chain formation. This chain formation is critical for ''in vivo'' function, in which formation of ubiquitin or SUMO chains results in cellular processes such as protein degradation <ref name="ubcsumocomplex"/>. | + | ''Ubc9'' is a part of the SUMOylation process. It is the enzyme responsible for ligating the SUMO to the protein. Depending on whether the reaction is done ''in vitro'', it will ligate the SUMO directly to the substrate, and if done ''in vivo'', the SUMO will be ligated to the conjugating enzyme and then put onto the substrate [2]. Initially, a thioester bond is formed between the SUMO and the E1 enzyme via an ATP-dependent reaction. The SUMO is then transferred to the active cystein of the E2, in this case, Ubc9. The SUMO is then ligated to a lysine side chain amino group of the substrate, during which an E3 enzyme may or may not be recruited. The use of E3 mediated transfer serves functions such as increasing substrate specificity <ref name="ubcsumocomplex"/>The Mutagenesis studies on ''Ubc9'' SUMO1 complex have shown that ''Ubc9H20D'' and ''SUMO1E67R'' mutation cause inhibition of noncovalent interaction between the two, and thus inhibit complex formation. A similar interaction is seen in the ''Ubc9''-SUMO2 complex <ref name="ubcsumocomplex"/>. Lack of noncovalent interaction between ''Ubc9'' and SUMO2 has been shown to inhibit SUMO2 chain formation. This chain formation is critical for ''in vivo'' function, in which formation of ubiquitin or SUMO chains results in cellular processes such as protein degradation <ref name="ubcsumocomplex"/>. |
== Disease == | == Disease == | ||
Revision as of 02:32, 25 February 2015
| |||||||||||
References
- ↑ 1.0 1.1 Tong H, Hateboer G, Perrakis A, Bernards R, Sixma TK. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin-conjugating system. J Biol Chem. 1997 Aug 22;272(34):21381-7. PMID:9261152
- ↑ 2.0 2.1 2.2 2.3 Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007 Jun 6;26(11):2797-807. Epub 2007 May 10. PMID:17491593