We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ubc9
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
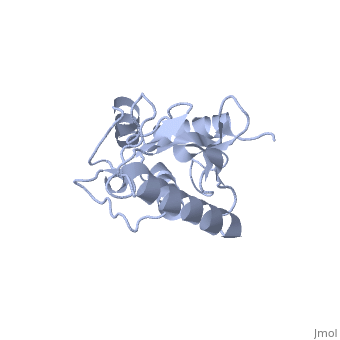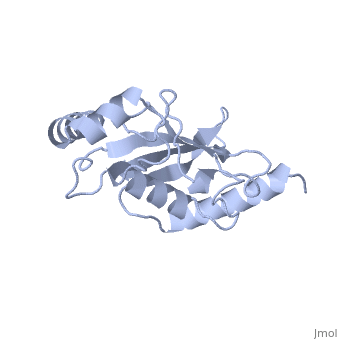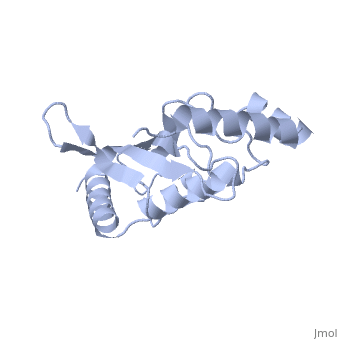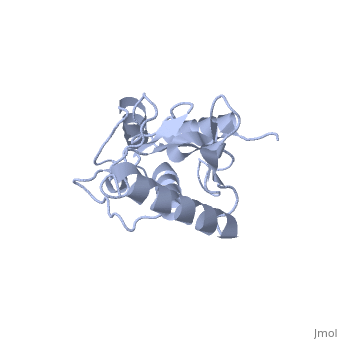
| - | Murine/human ''Ubc9'' exhibits a single domain structure consisting of both alpha helices and beta-pleated sheets (PDB: 1U9A). <scene name='69/694804/Cys93/6'>Cys93</scene> has been identified as the active residue and is located on a loop of amino acids (78-108) between beta sheet four and alpha helix two <ref name="crystal"/>. Crystal structure of the <scene name='69/694804/Noncovalent_ubc9_sumo1/2'>noncovalent interaction between Ubc9 and SUMO1</scene> (PDB: 2UYZ) have shown that the Ubc9 residues involved in this interaction are located at the end of helix one, on beta strand one, and along the following loop. These residues were shown to interact with the beta sheet of SUMO1 <ref name="ubcsumocomplex">PMID:17491593</ref>. | + | Murine/human ''Ubc9'' exhibits a single domain structure consisting of both alpha helices and beta-pleated sheets (PDB: 1U9A). <scene name='69/694804/Cys93/6'>Cys93</scene> has been identified as the active residue and is located on a loop of amino acids (78-108) between beta sheet four and alpha helix two <ref name="crystal"/>. Along with the formation of a thioester bond the SUMO, Ubc9 also interacts noncovelently with SUMO. Crystal structure of the <scene name='69/694804/Noncovalent_ubc9_sumo1/2'>noncovalent interaction between Ubc9 and SUMO1</scene> (PDB: 2UYZ) have shown that the Ubc9 residues involved in this interaction are located at the end of helix one, on beta strand one, and along the following loop. These residues were shown to interact with the beta sheet of SUMO1 <ref name="ubcsumocomplex">PMID:17491593</ref>. |
== Function == | == Function == | ||
| - | ''Ubc9'' is a part of the SUMOylation process. It is the enzyme responsible for ligating the SUMO to the protein. Depending on whether the reaction is done ''in vitro'', it will ligate the SUMO directly to the substrate, and if done ''in vivo'', the SUMO will be ligated to the conjugating enzyme and then put onto the substrate [2]. Initially, a thioester bond is formed between the SUMO and the E1 enzyme via an ATP-dependent reaction. The SUMO is then transferred to the active cystein of the E2, in this case, Ubc9. The SUMO is then ligated to a lysine side chain amino group of the substrate, during which an E3 enzyme may or may not be recruited. The use of E3 mediated transfer serves functions such as increasing substrate specificity <ref name="ubcsumocomplex"/> | + | ''Ubc9'' is a part of the SUMOylation process. It is the enzyme responsible for ligating the SUMO to the protein. Depending on whether the reaction is done ''in vitro'', it will ligate the SUMO directly to the substrate, and if done ''in vivo'', the SUMO will be ligated to the conjugating enzyme and then put onto the substrate [2]. Initially, a thioester bond is formed between the SUMO and the E1 enzyme via an ATP-dependent reaction. The SUMO is then transferred to the active cystein of the E2, in this case, Ubc9. The SUMO is then ligated to a lysine side chain amino group of the substrate, during which an E3 enzyme may or may not be recruited. The use of E3 mediated transfer serves functions such as increasing substrate specificity <ref name="ubcsumocomplex"/>. Noncovalent interaction between Ubc9 and SUMO have been shown to be critical in the formation of SUMO chains, upon which ''in vivo'' functions such as protein degradation signaling are dependent. Mutagenesis studies on ''Ubc9'' SUMO1 complex have shown that ''Ubc9H20D'' and ''SUMO1E67R'' mutation cause inhibition of noncovalent interaction between the two, and thus inhibit complex formation. A similar interaction is seen in the ''Ubc9''-SUMO2 complex <ref name="ubcsumocomplex"/>. Lack of noncovalent interaction between ''Ubc9'' and SUMO2 has been shown to inhibit SUMO2 chain formation <ref name="ubcsumocomplex"/>. |
== Disease == | == Disease == | ||
Revision as of 02:51, 25 February 2015
| |||||||||||
References
- ↑ 1.0 1.1 Tong H, Hateboer G, Perrakis A, Bernards R, Sixma TK. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin-conjugating system. J Biol Chem. 1997 Aug 22;272(34):21381-7. PMID:9261152
- ↑ 2.0 2.1 2.2 2.3 Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007 Jun 6;26(11):2797-807. Epub 2007 May 10. PMID:17491593