UBC13 MMS2
From Proteopedia
| Line 22: | Line 22: | ||
== Pathway for DNA Repair == | == Pathway for DNA Repair == | ||
[[Image:Slide2.jpg]] | [[Image:Slide2.jpg]] | ||
| + | Figure. 1. The effected protein PCNA is a DNA clamp that is monoubiquitinated by the Rad6-Rad18 complex. The monoubiquitinated PCNA is then polyubuiquitinated by the UBC13-Mms2-Rad5 complex. | ||
| + | |||
== Structural Highlights/Important Residues == | == Structural Highlights/Important Residues == | ||
Revision as of 23:15, 25 February 2015
|
Contents |
Summary
Ubc13 is an E2 ubiquitin conjugating enzyme that can form a heterodimer with Mms2 to function as a part of the Translesion DNA Synthesis Pathway (TLS). When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18(another E2 complex).
Function
Ubc13 functions as a heterodimer with Mms2, a structurally similar protein to Ubc13 that lacks the catalytic cysteine residue in the active site. The Ubc13 E2 complex with Mms2 functions primarily to enhance DNA repair from double stranded breaks. Mms2 bound to Ubc13 helps orient the ubiquitin molecule for proper ubiquitination of the K63 residue. Mms2 is considered a UEV protein (Ubiquitin-conjugating Enzyme Variant), because it lacks the catalytic cysteine residue necessary for proper thioester formation.
Regulation
The regulation of Ubc13 is controlled by the competitive binding of the two different UEV's, Mms2 and UEV1A. Ubc13 binding to Mms2 activates the DNA repair pathway, while Ubc13 binding to UEV1A activates the NF-kappaB pathway, a gene regulation pathway involved in DNA transcription factors. These two opposing pathways being downstream targets for Ubc13-bound complexes is the basis for regulation.
Coordinating Enzymes
- Another E2 complex, Rad6-Rad18, starts the process of DNA repair by monoubiquitinating PCNA near the replication fork of DNA. This DNA repair will arrest cell cycle progression until DNA repair is complete.
- UEV1A, another cofactor enzyme that binds to Ubc13, is thought to compete with Mms2 for binding to Ubc13. This is thought to be a regulatory mechanism for Ubc13 activity in the nucleus of cells.
- Several DNA polymerases such as rev1, pol eta, and pol zeta contain Ubiquitin-binding domains that recognize K164 polyubiquitination of PCNA.
- Rad5, an E3 RING (Really Interesting New Gene) protein, interacts with the Ubc13-Mms2 heterodimer in order to ligate the ubiquitin on the PCNA. Rad5, as well as Rad18 (RING proteins) are involved in the recruitment of Ubc13-Mms2 heterodimer formation.
Pathway for DNA Repair
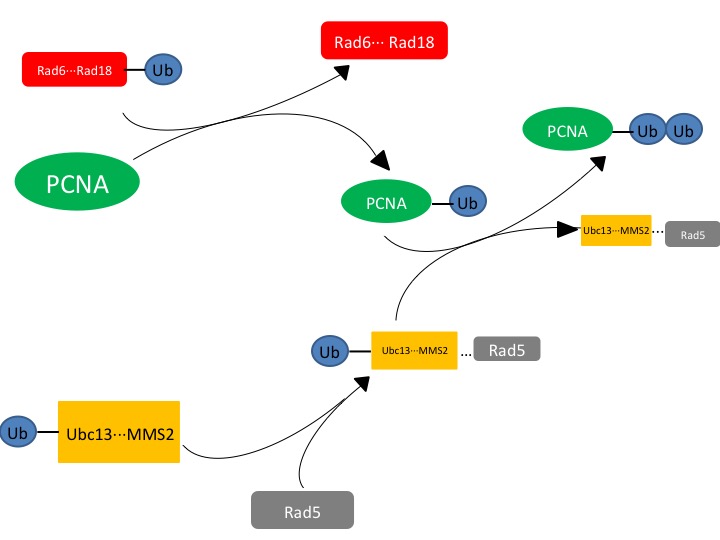 Figure. 1. The effected protein PCNA is a DNA clamp that is monoubiquitinated by the Rad6-Rad18 complex. The monoubiquitinated PCNA is then polyubuiquitinated by the UBC13-Mms2-Rad5 complex.
Figure. 1. The effected protein PCNA is a DNA clamp that is monoubiquitinated by the Rad6-Rad18 complex. The monoubiquitinated PCNA is then polyubuiquitinated by the UBC13-Mms2-Rad5 complex.
Structural Highlights/Important Residues
Ubc13 weights 17.6 kDA, and Mms2 is 16.8 kDA. The heterodimer is stable at high stalt concentrations (1 M), suggesting strong interactions between the two. Kd between the Ubc13 and Mms2 is 2 uM. Phe57 and Glu55 of Ubc13 interact with the N-terminal domain of Mms2 to ensure stable docking. Additionally, Arg70 hydrophobically interacts with an alpha helix of Mms2 in two places. Mms2’s Phe13 is inserted between Glu55, Phe57, and Arg70 of Ubc13 to create a hydrophobic pocket. It is therorized that Glu55 and Arg70 of Ubc13 are more important for recognition instead of stability[1].
Mechanism
The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism either occurs in a step-wise or concerted reaction.
References
[2] [3] [4] [5] [6] [7] [8] [9] [10]
- ↑ Pastushok L, Moraes TF, Ellison MJ, Xiao W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. J Biol Chem. 2005 May 6;280(18):17891-900. Epub 2005 Mar 4. PMID:15749714 doi:http://dx.doi.org/10.1074/jbc.M410469200
- ↑ Halas A, Podlaska A, Derkacz J, McIntyre J, Skoneczna A, Sledziewska-Gojska E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Mol Microbiol. 2011 May;80(3):786-97. doi: 10.1111/j.1365-2958.2011.07610.x. Epub, 2011 Mar 16. PMID:21362066 doi:http://dx.doi.org/10.1111/j.1365-2958.2011.07610.x
- ↑ Pastushok L, Moraes TF, Ellison MJ, Xiao W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. J Biol Chem. 2005 May 6;280(18):17891-900. Epub 2005 Mar 4. PMID:15749714 doi:http://dx.doi.org/10.1074/jbc.M410469200
- ↑ Andersen PL, Zhou H, Pastushok L, Moraes T, McKenna S, Ziola B, Ellison MJ, Dixit VM, Xiao W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. J Cell Biol. 2005 Aug 29;170(5):745-55. PMID:16129784 doi:http://dx.doi.org/10.1083/jcb.200502113
- ↑ Sato Y, Yamagata A, Goto-Ito S, Kubota K, Miyamoto R, Nakada S, Fukai S. Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem. 2012 Jul 27;287(31):25860-8. doi: 10.1074/jbc.M112.364752. Epub 2012, Jun 7. PMID:22679021 doi:http://dx.doi.org/10.1074/jbc.M112.364752
- ↑ Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002 Sep 12;419(6903):135-41. PMID:12226657 doi:10.1038/nature00991
- ↑ Moraes TF, Edwards RA, McKenna S, Pastushok L, Xiao W, Glover JN, Ellison MJ. Crystal structure of the human ubiquitin conjugating enzyme complex, hMms2-hUbc13. Nat Struct Biol. 2001 Aug;8(8):669-73. PMID:11473255 doi:10.1038/90373
- ↑ VanDemark AP, Hofmann RM, Tsui C, Pickart CM, Wolberger C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell. 2001 Jun 15;105(6):711-20. PMID:11440714
- ↑ Brusky J, Zhu Y, Xiao W. UBC13, a DNA-damage-inducible gene, is a member of the error-free postreplication repair pathway in Saccharomyces cerevisiae. Curr Genet. 2000 Mar;37(3):168-74. PMID:10794173
- ↑ McKenna S, Spyracopoulos L, Moraes T, Pastushok L, Ptak C, Xiao W, Ellison MJ. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. J Biol Chem. 2001 Oct 26;276(43):40120-6. Epub 2001 Aug 14. PMID:11504715 doi:http://dx.doi.org/10.1074/jbc.M102858200
Proteopedia Page Contributors and Editors (what is this?)
David A Taves, Michal Harel, Christopher Alexander Hudson, Nicholas R. Dunham
