We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 996
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
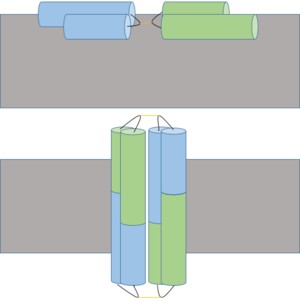
[[Image:Possible_Ectatomin_Mechanism_3.png|300px|left|thumb| A proposed insertion (above) and dimerization mechanism to form cation channel (below) of Ectatomin in the cell membrane (gray). Insertion occurs when the α and β subunits open at the hairpin hinge region (shown in black with yellow disulfide bonds), exposing internal hydrophobic residues which interact with hydrophobic lipid tails of the cell membrane. Pore formation occurs after dimerization, allowing ions to freely cross the membrane.]] | [[Image:Possible_Ectatomin_Mechanism_3.png|300px|left|thumb| A proposed insertion (above) and dimerization mechanism to form cation channel (below) of Ectatomin in the cell membrane (gray). Insertion occurs when the α and β subunits open at the hairpin hinge region (shown in black with yellow disulfide bonds), exposing internal hydrophobic residues which interact with hydrophobic lipid tails of the cell membrane. Pore formation occurs after dimerization, allowing ions to freely cross the membrane.]] | ||
| - | Ectatomin has several proposed mechanisms of action. The primary proposed mechanism involves the formation of a nonselective cation channel. In this mechanism, the α and β subunits open up, exposing the internal hydrophobic residues. The protein flattens while remaining attached at the hairpin hinge region. The now exposed hydrophobic residues nonselectively insert into plasma membranes. The inserted protein dimerizes, eventually forming a nonselective cation channel. | + | Ectatomin has several proposed mechanisms of action. The primary proposed mechanism involves the formation of a nonselective cation channel. In this mechanism, the α and β subunits open up, exposing the internal hydrophobic residues.<ref name="refone" /> The protein flattens while remaining attached at the hairpin hinge region. The now exposed hydrophobic residues nonselectively insert into plasma membranes.<ref name="refone" /> The inserted protein dimerizes, eventually forming a nonselective cation channel.<ref name="refthree" /> |
| - | For the second and third proposed mechanisms of action, Ectatomin has also been shown to inhibit kinases, specifically protein tyrosine kinase and protein kinase C, and Ca<sup>2+</sup> channels. Kinase inhibition would potentially allow Ectatomin to interfere with various components of signal transduction. Calcium channel inhibition would potentially allow Ectatomin to affect physiological processes such as contraction, neurotransmitter release and neuronal activity regulation. | + | For the second and third proposed mechanisms of action, Ectatomin has also been shown to inhibit kinases, specifically protein tyrosine kinase and protein kinase C, and Ca<sup>2+</sup> channels. Kinase inhibition would potentially allow Ectatomin to interfere with various components of signal transduction.<ref name="refthree" /> Calcium channel inhibition would potentially allow Ectatomin to affect physiological processes such as contraction, neurotransmitter release and neuronal activity regulation.<ref name="reffour" /> |
== Toxicology == | == Toxicology == | ||
| + | |||
| + | Ectatomin is one of the deadliest proteins known to man, alongside [https://en.wikipedia.org/wiki/Tetanospasmin Tetanospasmin] and the [https://en.wikipedia.org/wiki/Botulinum_toxin Botulinum toxin]. This protein attacks and forms pores in the plasma membrane where cations can freely move in and out of the cell. As the concentrations on either side of the cell reach equilibrium, the cells and the person effected with the toxin dies. The major cations effected is the calcium ion. Without the calcium gradient the muscles are not able to contract leaving the effected person paralyzed. Another major worry with free flowing cations reaching equilibrium is with potassium. Without a potassium gradient the heart will not be able to contract/beat leaving the person unable to push oxygen and nutrients throughout the body and to the brain. | ||
| + | |||
<ref name="refone">PMID: 7881269</ref> | <ref name="refone">PMID: 7881269</ref> | ||
Revision as of 00:14, 11 March 2015
Ectatomin (1eci)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Arseniev AS, Pluzhnikov KA, Nolde DE, Sobol AG, Torgov MYu, Sukhanov SV, Grishin EV. Toxic principle of selva ant venom is a pore-forming protein transformer. FEBS Lett. 1994 Jun 27;347(2-3):112-6. PMID:8033986
- ↑ 2.0 2.1 2.2 Pluzhnikov K, Nosyreva E, Shevchenko L, Kokoz Y, Schmalz D, Hucho F, Grishin E. Analysis of ectatomin action on cell membranes. Eur J Biochem. 1999 Jun;262(2):501-6. PMID:10336635
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Nolde DE, Sobol AG, Pluzhnikov KA, Grishin EV, Arseniev AS. Three-dimensional structure of ectatomin from Ectatomma tuberculatum ant venom. J Biomol NMR. 1995 Jan;5(1):1-13. PMID:7881269
- ↑ 4.0 4.1 Touchard A, Labriere N, Roux O, Petitclerc F, Orivel J, Escoubas P, Koh JM, Nicholson GM, Dejean A. Venom toxicity and composition in three Pseudomyrmex ant species having different nesting modes. Toxicon. 2014 Sep;88:67-76. doi: 10.1016/j.toxicon.2014.05.022. Epub 2014 Jun 11. PMID:24929139 doi:http://dx.doi.org/10.1016/j.toxicon.2014.05.022