Sandbox Reserved 427
From Proteopedia
(Difference between revisions)
| Line 63: | Line 63: | ||
A hydropathy plot for this receptor reinforces this fact, with a large span of the molecule shown again as trans-membrane alpha helices. | A hydropathy plot for this receptor reinforces this fact, with a large span of the molecule shown again as trans-membrane alpha helices. | ||
| - | -Show Hydropathy plot displaying opioid receptor as an amphipathic alpha helix | ||
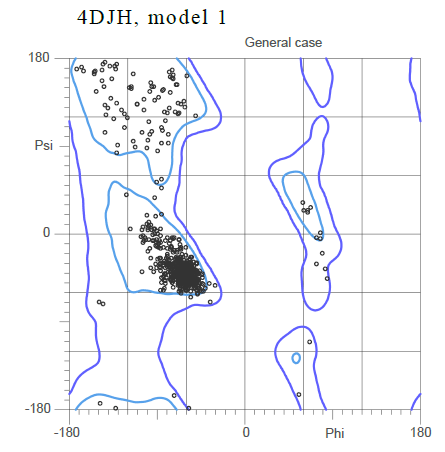
Based on the Ramachandran plot for the general case showing the standard phi and psi angle, it can be inferred that the majority of the secondary structure is composed <scene name='48/483884/Alphabeta/1'>mostly alpha helices</scene>, with the alpha helices shown in <font color='purple'>purple</font> and the beta sheets shown in <font color='yellow'>yellow</font>. | Based on the Ramachandran plot for the general case showing the standard phi and psi angle, it can be inferred that the majority of the secondary structure is composed <scene name='48/483884/Alphabeta/1'>mostly alpha helices</scene>, with the alpha helices shown in <font color='purple'>purple</font> and the beta sheets shown in <font color='yellow'>yellow</font>. | ||
Revision as of 23:43, 31 March 2015
Ásliding right into the DMs
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Kappa-Opioid Receptor
| |||||||||||